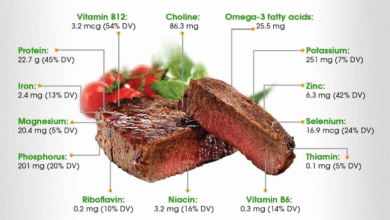
Thimerosal in Vaccines: CDC’s Latest Recommendations
Thimerosal in vaccines has long been a topic of public concern, as this mercury-based preservative has been used in multi-dose vaccines since the 1930s. In a recent meeting held by the CDC’s Advisory Committee on Immunization Practices (ACIP), the safety and recommendations for thimerosal-containing influenza vaccines were on the agenda. Despite worries about mercury in vaccines, it’s essential to note that health authorities, including the CDC, assert that thimerosal does not pose significant health risks. The American Academy of Pediatrics and various public health organizations have taken steps to reduce thimerosal in vaccines, particularly for children under six years old. However, the multi-dose flu vaccines still contain thimerosal, although single-dose versions free from this preservative are now available, as recommended by the CDC for both adults and children.
The discussion surrounding thimerosal, also known as thiomersal, has sparked ongoing debates about vaccine safety and efficacy. This mercury-containing agent has been employed in medical products to prevent bacterial growth, especially in multi-dose vaccine vials. Following the CDC recommendations, there has been a significant push to eliminate such preservatives from routine childhood vaccinations. As concerns about mercury exposure continue to influence public perception, it’s crucial to clarify the distinctions between thimerosal safety and the risks associated with other vaccine ingredients. As health professionals emphasize the necessity of vaccinations, understanding the role of preservatives like thimerosal is essential to foster informed discussions about immunization practices.
Understanding Thimerosal in Vaccines
Thimerosal, a mercury-containing preservative, has been a topic of discussion regarding its use in vaccines, particularly those administered in multi-dose vials. Historically, its role has been crucial in preventing bacterial and fungal contamination in vaccines since the 1930s. The Centers for Disease Control and Prevention (CDC) states that thimerosal has helped ensure the safety and efficacy of vaccines by maintaining sterility during storage. Despite its long-standing history, thimerosal has faced scrutiny as concerns about mercury exposure have emerged over the years, leading to extensive research on its safety and effects.
In response to public health concerns about thimerosal, significant actions were taken, particularly in the early 2000s. In 2001, it was agreed upon by major health organizations, including the American Academy of Pediatrics and the Public Health Service, to reduce or eliminate the use of thimerosal in vaccines recommended for children aged six and younger. Today, most vaccines no longer contain thimerosal; however, some formulations, especially multi-dose influenza vaccines, still include it. This complex history reflects the balancing act health agencies face between maintaining vaccine safety and addressing public concerns.
Frequently Asked Questions
What is thimerosal in vaccines, and why is it used?
Thimerosal in vaccines is a mercury-based preservative that has been used since the 1930s to prevent contamination by stopping the growth of bacteria and fungi in multi-dose vials. It helps ensure the safety and efficacy of vaccines by preventing microbial contamination during use.
Are there any safety concerns regarding thimerosal in vaccines?
Despite past concerns about mercury exposure, thimerosal in vaccines has been deemed safe by health agencies, including the CDC. Multiple studies have shown no evidence of health risks associated with the low doses of thimerosal used in vaccines.
What are the CDC recommendations regarding thimerosal in vaccines?
The CDC recommends that all adults and children 18 years and younger should receive seasonal influenza vaccines only in single-dose formulations that do not contain thimerosal. This recommendation aligns with efforts to reduce potential mercury exposure in the population.
Is thimerosal still present in vaccines today?
Thimerosal is still used in some multi-dose influenza vaccines, but there are alternatives available that are thimerosal-free. As of 2001, thimerosal was removed from most vaccines recommended for children aged 6 and younger.
How does thimerosal compare to other vaccine preservatives?
Thimerosal is one of several vaccine preservatives designed to prevent contamination. Unlike other preservatives used in medical products that may contain different compounds, thimerosal has undergone extensive evaluation, and its use in vaccines has established a strong safety record.
What types of vaccines still contain thimerosal?
Currently, thimerosal is mainly found in multi-dose vials of influenza vaccines, particularly those manufactured outside of the United States. Single-dose versions of vaccines are thimerosal-free and are recommended for use, especially for children and pregnant women.
What is the stance of health organizations on thimerosal safety?
Health organizations, including the CDC and the American Academy of Pediatrics, assert that thimerosal is safe in the low doses used in vaccines. They emphasize that data from numerous studies show no adverse health effects linked to thimerosal-containing vaccines.
Why was thimerosal removed from children’s vaccines?
Thimerosal was removed from children’s vaccines as a precautionary measure in 2001 to address growing public concerns about mercury exposure. The decision was part of a broader commitment by health agencies to enhance vaccine safety, despite no conclusive evidence of harm.
Can I request a thimerosal-free vaccine for my child?
Yes, parents can request thimerosal-free vaccine options for their children. Most healthcare providers can accommodate this request as single-dose formulations of recommended vaccines are typically without thimerosal.
What are the potential risks of thimerosal in vaccines?
Health authorities maintain that there are no identifiable risks associated with thimerosal in the doses found in vaccines. The consensus is that thimerosal is safe and that its levels do not pose health risks to the public.
| Key Point | Details |
|---|---|
| Agenda Inclusion | Thimerosal was on the agenda for a CDC meeting of the Advisory Committee on Immunization Practices on June 26. |
| Nature of Thimerosal | Thimerosal is a mercury-based preservative used since the 1930s in multi-dose vaccines to prevent contamination. |
| Concerns and Reductions | In 2001, thimerosal was largely removed from childhood vaccines with few exceptions due to concerns about mercury exposure. |
| Current Recommendations | CDC recommends that all adults and children under 18 receive thimerosal-free single-dose flu vaccines. |
| Safety Claims | The CDC maintains there is no evidence that thimerosal poses health risks and has a strong safety record. |
| Current Usage | Thimerosal is still present in some multi-dose flu vaccines, primarily outside the U.S. |
Summary
Thimerosal in vaccines has been a significant topic of discussion, particularly due to its inclusion in the CDC’s agenda regarding vaccine safety. Despite extensive use and historical concerns over mercury exposure, health agencies like the CDC reaffirm the safety of thimerosal. The gradual removal of thimerosal from vaccines, especially for children, reflects a commitment to addressing public concerns while maintaining effectiveness against contamination. With ongoing recommendations for thimerosal-free formulations, it is essential for parents to stay informed about the ingredients in vaccines to make safe choices for their health.