Men’s Multivitamins Recall Due to Undeclared Allergen

In a concerning development, a recall has been issued for certain men’s multivitamins due to undeclared allergens, specifically soy flour. This action, prompted by the Food and Drug Administration (FDA), highlights the potential danger for consumers with soy allergies, emphasizing the importance of allergen warnings in health supplements. The affected product, manufactured by MTN OPS LLC, is the Multi-V Men multivitamin, packaged in bottles containing 60 capsules, with approximately 7,546 bottles impacted. Classified as a Class II recall by the FDA, this situation raises awareness about the safety standards concerning men’s health supplements. As consumers become increasingly vigilant about their supplement choices, such recalls serve as critical reminders of the importance of clear labeling and transparency in multivitamins.
The recent safety concerns surrounding male dietary supplements have come to the forefront due to a widespread recall of multivitamins. Specifically, men’s health products have been flagged for containing unexpected ingredients that could be hazardous to individuals with particular allergies. Following rigorous FDA evaluations, these supplements were found to have undeclared components such as soy flour, which poses a risk for those with soy-related allergies. This incident reiterates the need for strict adherence to allergen guidelines in vitamin formulations. With a shift towards ingredient transparency, the landscape of men’s vitamins is evolving, compelling consumers to stay informed and cautious about the supplements they choose.
Understanding the Recent Recall of Men’s Multivitamins
In April 2025, the Food and Drug Administration (FDA) announced a recall of Multi-V Men multivitamins produced by MTN OPS LLC. This recall stemmed from the presence of undeclared soy flour, an allergen that could pose health risks, particularly to individuals with soy allergies. The recall impacted approximately 7,546 bottles of these multivitamins, raising serious concerns for consumers relying on these supplements for their daily health. With an expiration date of March 2026, the recalled vitamins were marketed for their ability to support men’s health, highlighting the necessity for vigilance when it comes to product labels and ingredient lists.
The FDA classified this recall as Class II, indicating that while the potential for serious health consequences is low, there is still the possibility of temporary or medically reversible effects. Consumers who may have purchased these products should check their shelves, as the health implications of ingesting undeclared allergens can be severe. It is crucial for manufacturers to maintain transparency in their product labeling to assure consumers that their health is not at risk, especially when it comes to essential supplements aimed at enhancing men’s health.
Impact of Undeclared Allergens in Dietary Supplements
The presence of undeclared allergens in dietary supplements like multivitamins poses a significant public health issue. Allergens such as soy flour, when not listed on the label, can lead to serious allergic reactions in sensitive individuals. This is particularly pressing because men who choose to incorporate multivitamins into their daily health regimen often do so to maintain optimal wellness and fortify their immune systems. As per FDA guidelines, all allergens must be clearly stated, as neglecting this can endanger consumers and undermine their trust in dietary supplements.
Furthermore, the fallout from such recalls extends beyond individual health risks; it can affect the entire supplement industry. Consumers may become skeptical of not only the implicated products but also similar health supplements on the market. This necessitates a call for increased regulatory scrutiny and rigorous testing among manufacturers to ensure that products labeled as safe truly meet such claims. Additionally, companies like MTN OPS must improve their oversight and quality control processes to avoid future instances that could harm their customers and jeopardize their brand reputation.
Multivitamins and Men’s Health: What You Need To Know
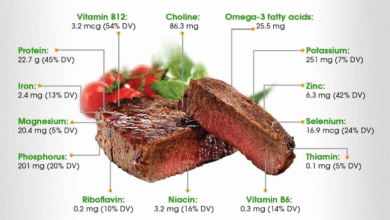
Men’s health supplements, particularly multivitamins, play a crucial role in fulfilling the nutritional gaps in men’s diets. Formulated specifically to meet men’s unique health needs, these supplements typically include essential vitamins and minerals such as calcium, vitamin B-12, and zinc. However, the discovery of undeclared allergens like soy flour in these supplements underscores the importance of thorough ingredient vetting for consumers. Men seeking to improve their health must prioritize supplements that are transparent regarding their ingredients to avoid potential allergic reactions.
Healthcare professionals often recommend incorporating multivitamins into a balanced diet, especially for men who may not get enough nutrients from food alone. Despite the benefits, the recent recall points to an underlying theme in the supplement industry—accountability and accuracy in labeling. As awareness grows regarding ingredient transparency, both manufacturers and consumers must advocate for rigorous testing and clear communication about product contents. Ensuring that the multivitamins consumed are free from allergens is essential for men’s overall health and safety.
How to Identify Safe Men’s Multivitamins
When choosing a multivitamin, knowing how to identify safe and quality products is vital, especially following incidents like the MTN OPS recall. Start by examining product labels for allergen declarations and ingredient lists. Always look for those that adhere to FDA regulations and ideally carry quality certifications from third-party testing organizations. These ensure that the vitamins not only meet safety standards but also contain the ingredients as advertised. Opting for brands renowned for their transparency in their formulation processes can further reduce the risk of exposure to undeclared allergens.
Additionally, it is advisable to stay informed about recent recalls and advisories issued by the FDA. Consumers can access the FDA’s website or sign up for alerts to keep track of any updates regarding potential issues with dietary supplements. Knowledge about allergens, such as soy flour, and their marked presence or absence in multivitamins can make a significant difference in making informed decisions. The aim is to safeguard health while still reaping the benefits of vitamins designed to enhance men’s vitality and wellbeing.
Navigating Allergens in Health Supplements
To navigate allergens in health supplements effectively, it’s crucial to be well-informed about common allergens and their potential health impacts. For men considering multivitamins, understanding the implications of allergens like soy flour becomes paramount, particularly for those with known sensitivities. Consumption of a product containing undeclared allergens could lead to serious health consequences and allergic reactions, reinforcing the need for diligence during supplement selection.
Consulting with healthcare providers can also aid in choosing the right vitamins tailored to individual health needs and dietary restrictions. In tandem, discussing supplement choices that are especially tailored to accommodate allergens or promote hypoallergenic formulations can offer men safer options. Awareness and communication about how to identify and avoid allergen-containing products can empower individuals to take charge of their health proactively.
The Role of the FDA in Supplement Safety
The FDA plays a crucial role in ensuring the safety and efficacy of dietary supplements, including men’s multivitamins. Through rigorous product evaluations and monitoring of recalls like the recent MTN OPS case, the FDA aims to protect consumers from potentially harmful products. The classification of recalls, such as this particular Class II recall, is part of a larger framework that underscores the FDA’s commitment to maintaining public safety regarding health supplements. Furthermore, the FDA mandates that manufacturers adhere to strict labeling requirements, which help consumers make informed choices.
However, the responsibility does not rest solely with regulatory agencies; manufacturers must also engage in rigorous quality control and transparency about their products. This dual approach—where the FDA and supplement companies ensure adherence to safety standards—fosters an environment where consumers can trust the integrity of the products they consume. As the market for men’s health supplements continues to expand, collaboration between regulators and companies becomes essential to uphold safety regulations and public trust.
Exploring Alternative Brands of Men’s Vitamins
Amid the recall of MTN OPS’s Multi-V Men, consumers may want to explore alternative brands of men’s vitamins that prioritize ingredient safety and allergen transparency. Options abound in the marketplace, with companies that are committed to rigorous testing and certification processes. Brands that actively promote their adherence to FDA standards and provide comprehensive labeling are often more reliable choices for men seeking to enhance their health without jeopardizing their safety.
Furthermore, researching ingredient sources and reviewing customer feedback can provide additional insights into the reliability of different supplement brands. Many consumers are turning to natural or organic options that emphasize whole-food vitamins, which may pose fewer risks concerning allergens. By broadening their research, consumers can discover alternatives that not only meet their nutritional needs but also align with their health philosophies.
Men’s Health Supplements: Trends and Innovations
The world of men’s health supplements is ever-evolving, with trends and innovations constantly reshaping consumer choices. Recent shifts toward more natural formulations without synthetic additives are gaining popularity, and brands are increasingly focusing on allergen-free products. As awareness of allergen sensitivities rises, manufacturers are adapting their formulations to cater specifically to men who seek supplements that support their health without risking exposure to allergens, such as soy flour.
Additionally, advancements in technology facilitate rigorous testing methods to ensure that multivitamins are free from contaminants and undeclared substances. The integration of consumer feedback into product development allows companies to refine their offerings, paving the way for a more personalized approach to men’s health supplements. As more consumers advocate for transparency and high-quality standards, the industry will likely continue to innovate and evolve, setting new benchmarks for safety and efficacy.
Tips for Staying Informed About Health Supplement Recalls
Consumers should take an active role in staying informed about health supplement recalls, particularly as they relate to men’s multivitamins. Regularly visiting the FDA’s website enables individuals to access up-to-date information on product warnings, recalls, and safety alerts related to dietary supplements. Signing up for newsletters or alerts from reputable health organizations can provide timely updates on specific products and brands, ensuring that consumers remain informed and vigilant.
Engaging with community forums or following health-related social media pages can also be beneficial. Discussions among consumers about recent experiences and findings can offer useful insights into product safety and reliability. Additionally, encouraging open dialogue and sharing experiences related to allergen exposure can foster awareness, helping others to avoid risky products. Ultimately, remaining informed constitutes a critical step in safeguarding health while navigating the vast market of men’s vitamins.
Frequently Asked Questions
What prompted the recent men’s multivitamins recall by MTN OPS?
The recent men’s multivitamins recall by MTN OPS was prompted by the discovery of undeclared soy flour in their Multi-V Men product. This allergen poses a risk to consumers with soy allergies, which led the FDA to classify the recall as Class II.
How many bottles of MTN OPS men’s multivitamins are affected by the recall?
A total of 7,546 bottles of MTN OPS men’s multivitamins have been recalled due to the undeclared allergen. These multivitamins are packaged in 60-capsule bottles and have a lot number of #012324177.
What are the health implications of consuming recalled men’s multivitamins?
Consuming the recalled men’s multivitamins may result in temporary or medically reversible health consequences for individuals with soy flour allergies. The FDA indicated that while serious adverse health consequences are unlikely, caution is advised.
What specific nutrients are included in the recalled Multi-V Men multivitamins?
The recalled Multi-V Men multivitamins contain essential nutrients such as calcium, zinc, vitamin C, vitamin B-12, and vitamin D, aimed at supporting men’s daily and immune health.
What expiration date should consumers check for the recalled men’s multivitamins?
Consumers should check for an expiration date of March 2026 on the recalled men’s multivitamins. This ensures that the affected bottles are identified in the recall.
Have any other MTN OPS men’s health supplements been included in this recall?
No, the recall specifically includes only the Multi-V Men multivitamins produced by MTN OPS. No other batches or products have been reported as affected.
What steps should I take if I have purchased the recalled men’s multivitamins?
If you have purchased the recalled men’s multivitamins from MTN OPS, it is advised to discontinue use immediately and contact the company for guidance on returns or exchanges.
How can consumers stay informed about future FDA multivitamin recalls?
Consumers can stay informed about future FDA multivitamin recalls by regularly checking the FDA website and subscribing to their recall alerts, which provide information on various health supplements including men’s multivitamins.
| Key Point | Details |
|---|---|
| Recall Announcement | Men’s multivitamins recalled due to undeclared allergen. |
| Company Involved | MTN OPS LLC, based in Utah. |
| Product Details | Recalled Multi-V Men multivitamins packed in 60-capsule bottles, lots affected: 7,546 bottles. |
| Undeclared Allergen | Soy flour, which could trigger allergies. |
| FDA Classification | Class II recall indicating potential for temporary health consequences. |
| Expiration Date | March 2026, Lot number: #012324177. |
| Nutritional Components | Contains calcium, zinc, vitamin C, B-12, D, and thiamin. |
| Target Demographic | Designed for men’s daily health and immune support. |
Summary
The recent men’s multivitamins recall highlights a significant health concern due to the presence of undeclared soy flour, which poses a risk to those with soy allergies. Produced by MTN OPS LLC, the recalled Multi-V Men multivitamins affect over 7,500 bottles. The FDA has labeled this as a Class II recall, reflecting a risk that may lead to temporary health effects. It’s crucial for consumers, especially those with allergies, to remain informed about such recalls to ensure their safety.