COVID Vaccine Recommendations: FDA Chief Calls for More Data
COVID vaccine recommendations have become a hot topic as experts weigh in on the need for updated guidelines in light of recent data. With the recent FDA vaccine approval for an updated mRNA vaccine, many are left wondering how this will impact the 2025-2026 winter season. As discussions unfold about the efficacy of COVID booster shots, the scrutiny surrounding the Novavax vaccine controversy adds a layer of complexity to the decision-making process. The CDC vaccination guidelines may also evolve, potentially narrowing the recommendations based on risk categories. As public confidence fluctuates, it is imperative to remain informed about the latest vaccination strategies to protect against COVID-19.
As we navigate the ongoing pandemic, the discourse surrounding COVID-19 immunization strategies remains crucial. The latest insights into winter vaccine options highlight the potential changes in how vaccines are recommended for the coming seasons. With the FDA’s recent actions regarding vaccine efficacy and safety, health authorities are reassessing how to best protect vulnerable populations. Additionally, the controversies surrounding specific vaccine types like Novavax illustrate the complexity of public health messaging. Understanding these different facets of vaccination recommendations is essential for individuals looking to make informed health choices.
FDA Vaccine Approval Concerns
The FDA’s current stance on COVID-19 vaccines, particularly in light of the updated versions for the winter, is one of cautious evaluation. FDA Commissioner Dr. Marty Makary has expressed uncertainty regarding the necessity of these vaccines, indicating that more robust data is required before any approvals can be confidently issued. This skepticism is significant; it implies that without substantial evidence supporting their efficacy, the vaccines cannot be recommended for widespread use. The delay in FDA approval highlights the ongoing debate about the safety and necessity of boosters and new formulations as they are developed.
Furthermore, the FDA’s decision-making process regarding vaccine approvals is under scrutiny. With the backdrop of public skepticism towards vaccine recommendations, especially among healthcare workers who previously opted out of booster shots, the agency’s credibility in navigating these recommendations is crucial. Public trust, which has been significantly challenged throughout the pandemic, must be rebuilt through transparent communication and solid empirical data that would support any forthcoming decisions on COVID vaccine recommendations.
Winter COVID Vaccine Updates
As we approach the winter season, health authorities, including the FDA and CDC, are facing critical decisions about the updated COVID vaccines—specifically, the mRNA vaccines and the revamped Novavax formulation. The urgency to develop vaccines that effectively counter the dominant variants is paramount, as these changes could impact overall public health policy. Heightened preparations are underway in anticipation of potential waves in infection rates during the colder months, and understanding the efficacy of these vaccines is key to mitigating risks to vulnerable populations.
In addition, the FDA’s approval process takes into account the evolving landscape of COVID-19 variants that continue to emerge. As new variants are identified, adjustments to vaccine formulations may be necessary to ensure that they remain effective. This adaptive approach underscores the importance of robust clinical trials and the need for real-time data that informs regulatory decisions. The CDC’s emerging guidelines will hinge on these dynamics, ultimately shaping the recommendations that healthcare providers will issue as winter approaches.
Evaluating COVID Booster Shots
The discussion surrounding COVID booster shots is increasingly complex, particularly with the FDA’s call for more comprehensive data before endorsing these additional vaccinations. Dr. Makary highlighted the public trust issues prevalent among healthcare workers and the broader community, indicating that many individuals are hesitant to receive boosters without clear evidence of their necessity and effectiveness. This skepticism not only complicates public health efforts but also reflects the urgent need for transparency in how data is presented regarding booster shots.
Moreover, the CDC is evaluating different strategies for recommending COVID boosters as we approach the 2025-2026 season. Options range from universal recommendations for all ages to more nuanced guidelines targeting high-risk groups. The discussion of these options emphasizes the need for a tailored approach that considers individual risk factors such as age and underlying health conditions, while also reassessing the evolving benefits of booster shots based on contemporary scientific evidence.
The Novavax Vaccine Controversy
The Novavax vaccine has sparked considerable debate, particularly regarding its approval status amidst questions surrounding its effectiveness in the current immunity landscape. Dr. Makary has raised concerns about the lack of comprehensive studies that include individuals with natural immunity, pointing out that the original research did not account for this crucial element. As population-level immunity increases due to previous infections and vaccinations, the relevance of the Novavax vaccine’s data must be reassessed to ensure it meets the current public health needs.
Furthermore, Novavax’s response to the FDA’s call for additional clinical trials reflects the ongoing tension within vaccine development—striking a balance between timely access to vaccines and the undeniable necessity for rigorous scientific evidence. The public and healthcare professionals alike are awaiting clearer guidance and outcomes from future studies to better understand Novavax’s role within the array of recent vaccine offerings. This situation highlights the importance of continued research and transparent communication regarding the efficacy and safety of all vaccine options.
CDC Vaccination Guidelines Revisions
As the landscape of COVID vaccination evolves, the CDC is actively revising its guidelines, which may influence recommendations for the upcoming season. The agency is exploring options ranging from universal vaccination policies to targeted recommendations for high-risk groups. These considerations are critical in ensuring the most vulnerable populations receive necessary protection while balancing the need for public buy-in.
In its upcoming guidelines, the CDC will also address the growing incidence of vaccine skepticism and the waning public trust in health recommendations. By providing clear, concise information and evidence-based guidelines, the CDC hopes to improve vaccination rates and encourage more individuals to participate in booster programs. The efficacy of the CDC’s strategy will ultimately depend on their ability to communicate risk and benefit effectively, thereby fostering a stronger public health response to COVID-19.
Understanding Public Trust in COVID Vaccines
Public trust is a crucial component in the effectiveness of vaccination campaigns. The skepticism that has emerged around COVID booster shots demonstrates the need for clarity and trustworthiness in health communications. As seen in recent statements by the FDA and CDC, experts are increasingly aware of the importance of providing clear, evidence-based recommendations regarding vaccine safety and effectiveness. Efforts must be made to address public concerns directly, ensuring that information about the vaccines is both accessible and understandable to all.
Furthermore, rebuilding trust within the community requires a concerted effort among health leaders, scientists, and policymakers. Engaging with the public through transparent dialogue and sharing ongoing research findings can help to enhance confidence in vaccination programs. The FDA’s ongoing efforts to gather comprehensive data on vaccines, coupled with a willingness to address public skepticism directly, can play a pivotal role in fostering a culture of trust that underpins public health initiatives.
Navigating the Future of COVID Vaccinations
Looking ahead, the future of COVID vaccinations hinges on the ability of health authorities to adapt to new variants and emerging data. The FDA and CDC’s roles will be crucial in establishing a responsive framework that can accommodate evolving evidence about vaccine efficacy and public health needs. This agility will be vital as both agencies analyze real-world data to determine which populations should receive vaccinations and under what circumstances.
Additionally, collaboration among governmental health organizations, pharmaceutical companies, and research institutions will be essential for providing the most effective vaccines. By fostering a robust network of information exchange, health authorities can address pressing questions about front-line treatment and vaccine strategies—better preparing for potential future surges in COVID-19 cases. The ongoing dialogue surrounding vaccine recommendations will play a significant role in shaping how society navigates these challenges.
Impact of Healthcare Workers’ Vaccination Rates
Healthcare workers’ vaccination rates play a critical role in shaping public perception and adherence to vaccination guidelines. The hesitation observed among some in the healthcare field can influence broader community attitudes towards vaccines. With their unique position at the forefront of public health, healthcare professionals carry the responsibility of modeling positive vaccine behaviors. The trust placed in these individuals can significantly impact community vaccination rates, either by encouraging or discouraging uptake based on their own choices.
To combat this issue, health organizations must prioritize educational initiatives aimed at healthcare workers themselves. Providing them with access to reliable data and resources can empower them to make informed decisions about their health and that of their patients. It is equally important for health authorities to address the concerns of healthcare workers directly—acknowledging their experiences and providing transparent information about vaccine safety and effectiveness can help to rebuild trust and ensure a unified approach to vaccinations across the board.
The Importance of Continuous Research in Vaccines
Continuous research is essential in the ever-evolving landscape of COVID vaccines. The FDA and CDC rely heavily on the latest scientific studies to underpin their recommendations and guidelines. As new variants arise and further data become available, the landscape regarding the efficacy and safety of vaccines will shift, necessitating an adaptive approach to vaccination strategies. This ongoing investigation helps provide clarity on which vaccines, including booster shots, are most appropriate for different demographics.
Moreover, researchers and public health officials must prioritize transparency in sharing research findings with the public. Engaging the community in discussions about the importance of vaccine research can foster a positive perception, demonstrating the significant efforts made to ensure safety and efficacy. As the pandemic presents new challenges, a commitment to continuous research will be vital in reassuring the public about their vaccination decisions.
Frequently Asked Questions
What are the latest COVID vaccine recommendations from the FDA?
The FDA has recently approved updated mRNA COVID vaccines, alongside the Novavax vaccine, aimed at protecting against current variants for the winter 2024-2025 season. However, FDA Commissioner Dr. Marty Makary has expressed uncertainty about the necessity and effectiveness of these vaccines, highlighting the need for more data before full endorsement.
How does the CDC vaccination guideline impact COVID booster shots?
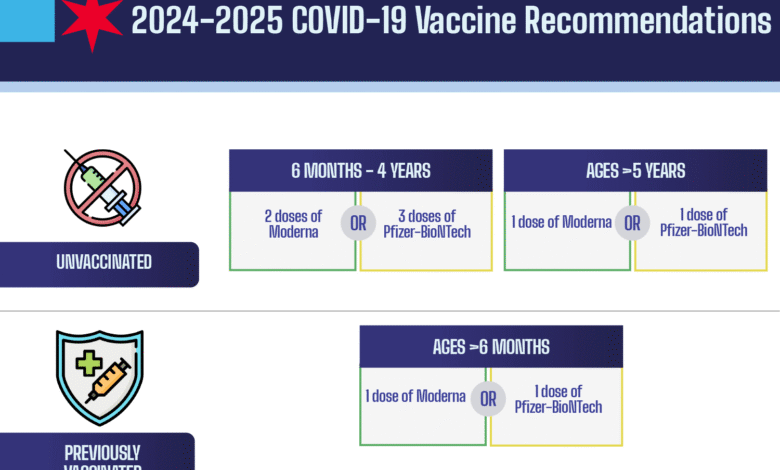
The CDC is considering revising its vaccination guidelines for COVID boosters for the 2025-2026 season. Possible changes include a universal recommendation for all individuals age six months and older or risk-based recommendations primarily for high-risk groups, emphasizing a more tailored approach based on factors like age and underlying conditions.
What is the controversy surrounding the Novavax vaccine in COVID vaccine recommendations?
The Novavax vaccine has sparked controversy due to concerns about insufficient data supporting its effectiveness, especially considering its previous study excluded individuals with natural immunity. The FDA is requesting additional clinical trials to validate its current applicability and effectiveness in the face of widespread population immunity.
Why is there skepticism about winter COVID vaccine recommendations for 2025?
Skepticism regarding winter COVID vaccine recommendations for 2025 stems from calls for more solid data and concerns about public trust in booster shots, especially given that many healthcare workers opted out of vaccinations last season. The FDA chief has indicated that thorough research is essential before making definitive recommendations.
What options is the CDC considering for future COVID vaccine recommendations?
The CDC is exploring various options for future COVID vaccine recommendations, including maintaining a universal vaccine policy for all ages, limiting recommendations to high-risk groups, or adopting age-based recommendations that transition to universal guidelines for individuals aged 65 and older, based on risk factors.
| Key Point | Details |
|---|---|
| FDA’s Current Evaluation | The FDA has not yet approved the COVID-19 vaccine for the upcoming winter season, expressing the need for more data. |
| Concerns About Data | FDA Commissioner Dr. Marty Makary emphasized a lack of solid data to support new COVID vaccine recommendations. |
| Public Trust Issues | Many healthcare workers opted not to get COVID boosters last season due to trust issues. |
| Novavax Vaccine Concerns | There is skepticism regarding Novavax’s efficacy, with calls for research on its performance against current variants. |
| CDC’s Vaccine Recommendations | The CDC is considering narrowing its recommendations, including options for universal vaccinations or focusing on high-risk groups. |
Summary
COVID vaccine recommendations are currently under intense scrutiny as FDA officials demand more data before approval for the winter vaccination season. With concerns over public trust and the adequacy of available research, healthcare leaders are debating the necessity and scope of booster shots, particularly for the 2025-2026 season. The evolving discourse suggests a potential shift towards more targeted vaccination strategies aiming to enhance efficacy and address the diverse needs of the population.