COVID-19 Vaccine for Children: AAP’s Latest Recommendations
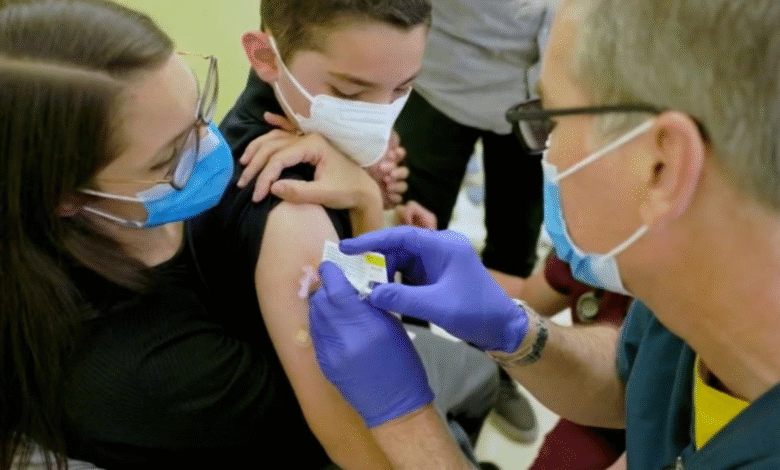
The COVID-19 vaccine for children has become a focal point of discussion as health authorities navigate the evolving landscape of pediatric health. Despite the recent CDC guidance that no longer recommends the COVID-19 vaccine for children, the American Academy of Pediatrics (AAP) stands firmly against this decision. In its latest children’s immunization schedule, the AAP emphasizes the importance of vaccinating infants and children aged 6 to 23 months, citing their higher risk for severe COVID-19 outcomes. According to AAP’s child vaccination guidelines, parents can opt for a COVID-19 vaccine for children aged 6 months and older, ensuring that they receive essential protection. The focus on COVID-19 vaccine safety remains pivotal as medical professionals strive to balance public health recommendations with the needs of families seeking security for their young ones.
When considering pediatric health measures, the discussion around the coronavirus vaccine for younger populations takes center stage. Recent shifts in recommendations by health organizations such as the CDC and AAP highlight the complexities involved in the vaccination landscape for children. The importance of ensuring immunity against not just COVID-19, but a range of vaccine-preventable diseases, underscores the need for informed decisions by parents and guardians. As we explore child health vaccination protocols, the focus on safeguarding our youth through immunizations—especially during a pandemic—remains critical. The dialogue surrounding these vaccine options illuminates the ongoing efforts to safeguard children’s health in our ever-changing world.
Understanding the AAP Vaccine Recommendation for COVID-19
The American Academy of Pediatrics (AAP) has made a significant statement regarding COVID-19 vaccination for children, particularly highlighting the need for a tailored approach based on individual health circumstances. Their recommendations emphasize that infants aged 6 to 23 months, who are at heightened risk for severe COVID-19, should receive the vaccine. This directive is in stark contrast to the recent guidance from the CDC, which no longer advocates for the vaccine in this demographic. Such variations underscore the need for parents to stay informed on the latest vaccine recommendations and to engage actively with healthcare providers about their children’s health.
According to the AAP, all children aged 6 to 23 months are recommended to receive the COVID-19 vaccine to protect against potential severe health outcomes. Moreover, the AAP suggests that children and teens aged 2 years and older, who either are at high risk or live with individuals who are more vulnerable to severe COVID-19 disease, receive a single age-appropriate dose. This risk-based strategy reflects a growing trend in child vaccination guidelines that prioritize protecting those at higher risk while providing parents with the option to vaccinate their healthy children.
The CDC’s Guidance on COVID-19 Vaccine and its Implications
The Centers for Disease Control and Prevention (CDC) has shifted its approach to COVID-19 vaccination for children, advocating for shared clinical decision-making rather than a blanket recommendation. This means that healthcare providers are encouraged to discuss the benefits and risks of vaccination with parents. This approach has raised questions among many parents about how to navigate the rapidly evolving landscape of child vaccination guidelines, especially in light of the varied implications of COVID-19 for children’s health.
The CDC’s updated guidance indicates that, while the organization no longer includes COVID-19 vaccines in the routine immunization schedule for healthy children and pregnant women, they still allow for vaccinations if requested by parents for children aged 6 months and older. This emphasis on a more customized dialogue between the caregiver and healthcare provider represents a departure from universal recommendations and highlights the importance of personalized medical advice in the context of child vaccination.
COVID-19 Vaccine Safety: Expert Insights and Recommendations
The topic of COVID-19 vaccine safety remains at the forefront of discussions, especially regarding children. Experts like Sean O’Leary, M.D., from the AAP, affirm the necessity of COVID-19 vaccination, particularly for vulnerable demographics including young children and those with underlying medical conditions. The AAP’s position hinges on the fact that the hospitalization rates for these groups are alarming, similar to those for other diseases that are preventable through vaccination. This alarming statistic reinforces the need for vigilance and preventative measures, including vaccination.
Furthermore, the AAP articulates that the COVID-19 vaccine has undergone rigorous testing and monitoring to ensure it meets safety standards before administration to children. Parents are encouraged to consider not only the safety data but also the context of their child’s health background when evaluating vaccination options. By reinforcing this commitment to safety and efficacy, the AAP aims to bolster confidence in public health initiatives concerning children’s immunization schedules.
Key Changes in the Children’s Immunization Schedule
The AAP has incorporated several pivotal adjustments in its latest immunization schedule for children, which now notably includes the COVID-19 vaccines alongside other critical vaccinations like those for flu and respiratory syncytial virus (RSV). These updates are designed to align current public health concerns with children’s health needs, ensuring that they receive comprehensive protection against infectious diseases. The inclusion of the COVID-19 vaccine reflects the ongoing evolution of pediatric healthcare guidelines in response to emerging health threats.
In addition to the COVID-19 vaccine recommendations, the AAP’s new schedule details other significant updates, such as the introduction of the pentavalent meningococcal vaccine and the adjustment of the starting age for the human papillomavirus vaccine. The review of these guidelines also includes the removal of certain vaccinations, such as a hepatitis vaccine that is no longer available. Such changes emphasize the necessity for ongoing assessment of children’s immunization schedules, which must be responsive to both epidemiological data and public health research.
Engaging Parents in COVID-19 Vaccination Decisions
As new guidance emerges surrounding COVID-19 vaccinations, parent engagement becomes increasingly critical. The dialogue about whether to vaccinate children now often revolves around individual risk assessments rather than broad recommendations. This shift empowers parents to make informed choices about vaccination for their children, based on discussions with their healthcare providers that take into consideration the child’s health history and family circumstances.
By fostering an environment where parents can freely express their concerns and preferences, healthcare professionals can ensure that decisions about COVID-19 vaccinations are both informed and aligned with each family’s values. This approach helps to establish a collaborative relationship which is pivotal during uncertain times, ultimately aiming for the best health outcomes for children.
Continuity of Care: Addressing All Vaccinations for Children
While the focus may be on COVID-19 vaccinations due to its significance in public health, the AAP emphasizes that children’s overall immunization schedules must remain a priority. Besides COVID-19, various vaccines protect against several diseases ranging from measles to hepatitis. Parents are urged to ensure their children keep up with these routinely scheduled immunizations, as these play a crucial role in maintaining herd immunity and preventing outbreaks of vaccine-preventable diseases.
Continuity of care is critical as families navigate changing recommendations for the COVID-19 vaccine. Health care providers can leverage the opportunity to reinforce the importance of adhering to the complete immunization schedule while discussing the nuances of new guidelines. By maintaining the focus on all vaccines, parents can be encouraged to prioritize long-term health in children, fostering a holistic approach to pediatric health care.
The Importance of Pediatric Vaccination in Current Context
In light of global health challenges, the importance of pediatric vaccination cannot be understated. Vaccines are instrumental in preventing diseases that have historically impacted children’s health across various regions. Despite changes in the CDC’s recommendations regarding the COVID-19 vaccine, the consensus remains clear: vaccination is an essential aspect of public health that safeguards not only individual children but the population at large.
Parents should actively participate in vaccination conversations, assessing the evidence provided by health professionals and understanding the implications of these recommendations for their children. With a proactive approach, families can contribute to communal health and safety by ensuring their children are adequately vaccinated, which remains a cornerstone of public health policy.
Deciphering the Differences Between AAP and CDC Guidelines
The discrepancies between the AAP and CDC guidelines regarding COVID-19 vaccinations highlight the complexities within pediatric healthcare. The AAP advocates for a more protective recommendation for younger children, emphasizing the vaccine’s role in mitigating the severity of illness. In contrast, the CDC’s current guidance leans towards individualized assessments, which may generate confusion among parents trying to navigate the best path for their children’s health.
Understanding these differences is vital for parents as they seek to make informed choices. Engaging in discussions with healthcare professionals can help clarify these guidelines, allowing parents to tailor their approach to vaccination based on their children’s health needs and existing public health advice. Such informed decision-making is crucial in adapting to the ever-evolving landscape of pediatric health advisories.
Future Directions in Child Vaccination Research and Policy
As the landscape of pediatric vaccination evolves, ongoing research and policy discussions remain pivotal for improving public health outcomes. Incorporating data from current vaccination effectiveness studies, particularly concerning COVID-19, helps shape future guidelines and immunization schedules. Continuous re-evaluation of the risks and benefits associated with vaccines is essential to ensure they meet the changing needs of the population.
Furthermore, stakeholder input, including voices from parents, healthcare providers, and public health experts, is crucial in the future direction of vaccine policy. This collaborative approach can help to enhance public confidence in vaccinations, address concerns about safety and efficacy, and ultimately contribute to achieving higher vaccination rates. By working together, we can foster a healthier environment for children, safeguarding their futures against infectious diseases.
Frequently Asked Questions
What is the AAP’s recommendation on the COVID-19 vaccine for children?
The American Academy of Pediatrics (AAP) recommends a COVID-19 vaccine for all children aged 6 to 23 months to protect them from severe illness. Additionally, children and teens aged 2 and older at high risk for severe COVID-19 should receive a single dose of the age-appropriate vaccine if they have never been vaccinated before. The AAP also supports vaccination for children aged 2-18 based on parental request.
How does the CDC guidance on COVID-19 vaccine for children differ from the AAP’s recommendations?
The CDC’s guidance on the COVID-19 vaccine for children emphasizes shared clinical decision-making. While the AAP recommends vaccination for specific age groups and at-risk children, the CDC allows parents and healthcare providers to discuss vaccination benefits and risks for individual children aged 6 months and older, leading to a more tailored approach.
What are the child vaccination guidelines regarding COVID-19 vaccination?
Child vaccination guidelines regarding COVID-19 state that all children aged 6 months and older may receive the COVID-19 vaccine, with the specifics depending on individual health circumstances and parental preferences. The AAP encourages vaccination for young children at higher risk and allows for parents to choose vaccination for healthy children aged 2-18.
Is the COVID-19 vaccine safe for children according to current recommendations?
Yes, according to the AAP and CDC guidance, the COVID-19 vaccine is considered safe for children. The AAP has stated that infants and children aged 6 to 23 months are at high risk for severe COVID-19, hence recommending vaccination to prevent serious illness. Parents are encouraged to discuss potential risks and benefits with their healthcare provider.
What should parents consider before vaccinating their children against COVID-19?
Parents should consider their child’s health status, age, and any underlying conditions when deciding on the COVID-19 vaccine. It’s crucial to consult with a healthcare provider to understand the benefits of vaccination in relation to their child’s specific circumstances and the current recommendations from organizations like the AAP and CDC.
Are there any recent updates to the children’s immunization schedule regarding the COVID-19 vaccine?
Yes, the AAP’s latest immunization schedule includes COVID-19 vaccines, contrasting with the CDC’s recent approach that recommends shared decision-making. The AAP continues to endorse COVID-19 vaccination for certain age groups while providing guidance for healthy children seeking vaccination.
| Key Point | Details |
|---|---|
| CDC Recommendations | The CDC no longer universally recommends the COVID-19 vaccine for children. |
| AAP Opposition | The AAP continues to recommend COVID-19 vaccinations for young children, especially those at high risk. |
| Vaccination Age Group | Infants and children aged 6 to 23 months are advised to receive the vaccine. |
| High-Risk Recommendations | AAP recommends vaccination for children aged 2 and older who are at high risk. |
| Parental Choice | Children aged 2-18 may receive the vaccine if requested by parents. |
| Shared Decision-Making | CDC suggests clinical decision-making involving parents and healthcare providers for vaccination. |
Summary
The COVID-19 vaccine for children has become a topic of debate, especially with the recent CDC guidance shifting toward a more individualized approach. Although the CDC no longer recommends a routine COVID-19 vaccination for all children, the American Academy of Pediatrics (AAP) highlights its importance, particularly for infants and young children at higher risk. The AAP advocates for shared decision-making between parents and healthcare providers regarding vaccination, emphasizing the importance of protection against severe illness caused by COVID-19. This evolving conversation reflects differing opinions on public health strategies for children’s vaccination.