Counterfeit Ozempic: FDA Warns of Fake Medications
Counterfeit Ozempic medications are causing alarm in the U.S. as the FDA has issued a critical warning about the presence of fake Ozempic drugs in the drug supply chain. The FDA revealed that several hundred counterfeit semaglutide injection units were distributed outside the authorized Novo Nordisk network, raising serious concerns about Ozempic safety. This recent development highlights the dangers associated with unregulated medications, particularly as patients seek affordable alternatives amid rising costs. Health officials have urged both patients and healthcare providers to verify lot and serial numbers before using these medications, as using Ozempic counterfeit medications can lead to potentially harmful health risks. As the FDA continues to investigate, it’s essential to stay informed on the safety and authentication of medications like Ozempic.
The emergence of counterfeit medications, specifically imposters of the popular diabetes treatment, highlights a growing risk for patients relying on semaglutide injections. These fake Ozempic variants not only compromise personal health but also challenge the integrity of the pharmaceutical supply chain. As health authorities like the FDA ramp up their efforts to thwart these unauthorized drugs, it’s vital for consumers to remain vigilant. Patients seeking weight loss or diabetes management through injectable treatments should be cautious, ensuring they access genuine products from reputable sources. Understanding the implications of utilizing counterfeit Ozempic can empower patients to make safer choices when it comes to their health.
The Rising Concern of Counterfeit Ozempic Medications
The alarming emergence of counterfeit Ozempic medications has raised serious health concerns across the United States. Following a recent FDA notification, hundreds of units of counterfeit semaglutide injections were identified within the drug supply, presenting a significant risk to patients relying on these medications for managing type 2 diabetes and obesity. The FDA’s proactive response to seize these unauthorized products is critical in protecting public health and ensuring that patients receive genuine and effective treatments.
Health officials urge individuals and healthcare providers to be vigilant when obtaining Ozempic medications, particularly those purchased through non-regulated channels. Counterfeit drugs can compromise treatment efficacy and pose serious health risks due to the lack of quality control in their manufacturing. The FDA is currently investigating the counterfeit products to evaluate their safety, and patients are encouraged to check the lot and serial numbers of their medications to avoid exposure to these potentially harmful substances.
Understanding the Dangers of Fake Ozempic Drugs
Fake Ozempic drugs, often manufactured in unregulated environments, can greatly endanger patient safety. The FDA’s investigation into counterfeit Ozempic highlights the risks associated with purchasing medications from questionable sources, including online retailers. According to experts, these counterfeit medications lack the necessary quality assurance, rendering them impostors that may contain dangerous or ineffective ingredients.
Dr. Brett Osborn emphasizes the importance of acquiring semaglutide injections only through licensed pharmacies and valid prescriptions to avoid counterfeit variants. The consequences of using fake Ozempic drugs can be severe, potentially leading to adverse health events that could have been prevented with the proper pharmaceutical-grade treatments. Patients should always consult healthcare professionals regarding their medications to prioritize safety and efficacy.
FDA Warnings on Counterfeit Ozempic: What You Need to Know
The FDA has issued critical warnings regarding the detection of counterfeit Ozempic drugs, indicating a need for public awareness about the risks associated with these medications. The agency specifies details such as the counterfeit lot number PAR0362 and serial numbers starting with ‘51746517,’ advising against their use. This vigilant monitoring underscores the FDA’s commitment to patient safety and the integrity of drug supply chains in the United States.
Moreover, the fact that adverse events associated with counterfeit products have been reported highlights the potential dangers of using unauthorized medications. Patients must be informed about the risks that come with counterfeit drugs, including the uncertainty about the ingredients and manufacturing practices behind these substitutes. It is imperative for individuals to only trust medications prescribed by licensed healthcare professionals to mitigate these hazards.
The Importance of Genuine Ozempic for Patient Safety
Genuine Ozempic, a semaglutide injection, plays a crucial role in the effective management of type 2 diabetes. Unlike counterfeit variations, authentic products undergo rigorous testing and adhere to stringent manufacturing standards that ensure their safety and efficacy. Patients using genuine Ozempic benefit from scientifically validated formulations that are essential for controlling their health conditions.
The presence of counterfeit Ozempic drugs in the market creates unnecessary health risks for patients, including the potential for severe side effects or ineffective treatment. Healthcare providers consistently stress the importance of sourcing medications from legitimate channels to ensure that patients receive the highest quality of care. Emphasizing the need for authenticity in prescription medications is vital in safeguarding patient health and preventing adverse drug events.
Navigating the Ozempic Market: Tips for Patients
With the increasing prevalence of counterfeit medications, patients must navigate the Ozempic market with caution. It is recommended that individuals only purchase Ozempic from licensed pharmacies and verify the credentials of the dispensary. Patients should also check for proper labels, expiration dates, and lot numbers, as these details are essential indicators of authenticity.
Furthermore, consulting with healthcare professionals before starting any medication, including Ozempic, is paramount. Medical experts can provide guidance on sourcing reputable medications and help patients understand the potential risks associated with counterfeit drugs. Prioritizing informed choices is key to ensuring effective treatment and maintaining overall health.
Legal Implications Surrounding Counterfeit Ozempic
The circulation of counterfeit Ozempic raises significant legal implications that extend beyond patient danger. The FDA’s ongoing investigation into these counterfeit drugs also seeks to address the criminal enterprises behind their production and distribution. Law enforcement agencies are involved in efforts to dismantle networks that profit from counterfeit medications and endanger public health.
Additionally, pharmaceutical companies like Novo Nordisk are taking a stand against the counterfeit market by increasing surveillance and enhancing consumer education about the dangers of fake Ozempic. The legal efforts aim to hold accountable those who attempt to exploit patients seeking affordable healthcare solutions while compromising safety standards. Raising awareness and understanding of these legal ramifications is an essential step in eliminating counterfeit medications from the market.
How to Identify Safe Ozempic Products
Identifying safe Ozempic products involves several key practices that patients should adopt when considering their medication options. Firstly, always ensure that the Ozempic is acquired through a licensed healthcare provider or a reputable pharmacy that is verified and inspected. This step is crucial in avoiding counterfeit products that may pose significant health risks.
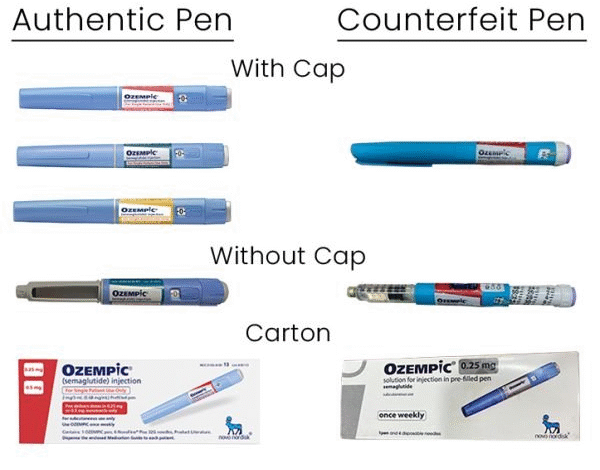
Additionally, patients should familiarize themselves with the packaging and labeling of genuine Ozempic medications. Authentic products display specific identifiers including lot numbers and barcodes, whereas counterfeit versions may lack this critical information or present discrepancies. Patients are encouraged to educate themselves on what to look for, allowing them to make safer choices regarding their medications.
The Role of Healthcare Providers in Preventing Counterfeit Ozempic
Healthcare providers play an essential role in the prevention of counterfeit Ozempic usage among their patients. By staying informed about the latest trends in counterfeit medications and understanding the potential risks associated with fake drugs, providers can better educate their patients on safe practices for acquiring their medications. This education not only helps patients make informed decisions but also instills confidence in the legitimacy of the medications they are prescribed.
Moreover, healthcare professionals should consistently advocate for the use of authorized pharmaceutical channels when discussing treatment options with patients. This advocacy is critical in mitigating the impact of counterfeit drugs and ensuring that patients receive effective and safe treatments. Building a trustworthy relationship between healthcare providers and patients can lead to better adherence to treatment plans and improved health outcomes.
Educational Resources for Patients on Ozempic Safety
Patients seeking reliable information on Ozempic safety and the risks of counterfeit medications can benefit from various educational resources. The FDA website offers comprehensive guidelines on recognizing counterfeit drugs and provides updates on safety alerts. Additionally, organizations dedicated to diabetes and obesity management often publish newsletters and articles that inform patients about treatment strategies and the importance of safe medication sourcing.
Moreover, direct communication with healthcare providers can serve as a valuable resource for patients. Engaging in honest discussions about their treatment options allows patients to gain insights into the significance of using genuine Ozempic and the dangers of counterfeit products. Utilizing these educational platforms equips patients with the knowledge needed to make safer health decisions and navigate the complexities of medication procurement.
Frequently Asked Questions
What should I know about counterfeit Ozempic medications?
Counterfeit Ozempic medications pose significant health risks as they are not manufactured or distributed through the authorized supply chain. The FDA has warned consumers about fake Ozempic drugs circulating in the U.S., specifically noting the lot PAR0362 with a serial number beginning with 51746517 should not be used. Always ensure you’re getting Ozempic from licensed pharmacies.
How can I identify fake Ozempic drugs?
To identify counterfeit Ozempic, check the packaging, lot number, and serial numbers against FDA warnings. Products labeled with lot number PAR0362 and serial numbers beginning with 51746517 are confirmed counterfeit and should not be used. Purchasing Ozempic through unregulated channels increases the risk of obtaining fake medications.
What are the dangers of using counterfeit Ozempic?
Using counterfeit Ozempic medications can lead to serious health complications due to unknown ingredients and lack of quality control. These fake drugs are often produced in non-medical environments, making them potentially dangerous. Always consult your healthcare provider and use medications prescribed by licensed professionals to ensure safety.
What actions has the FDA taken regarding counterfeit Ozempic?
The FDA has actively seized counterfeit Ozempic products identified in the U.S. drug supply as of April 9, 2025. They continue to test these seized products for safety and are investigating the supply chain to protect consumers from fake Ozempic medications.
How can I ensure the safety of my Ozempic prescription?
To ensure the safety of your Ozempic prescription, only obtain it from licensed pharmacies with a valid prescription from a healthcare provider. Avoid purchasing Ozempic or its variants online from unregulated sources, as these could be counterfeit and harmful.
What are the FDA’s warnings regarding Ozempic safety?
The FDA has issued warnings about the safety risks associated with counterfeit Ozempic medications. They emphasize the importance of checking lot and serial numbers, as well as ensuring your medication comes from a legitimate source to avoid the dangers posed by fake Ozempic drugs.
What are the known effects of counterfeit Ozempic on health?
The known effects of counterfeit Ozempic can vary widely due to the lack of regulation in their production. Users may experience adverse reactions that are not associated with genuine Ozempic. The FDA is monitoring reports of adverse events related to counterfeit medications and stresses the importance of getting prescribed medications from authorized sources.
| Key Point | Details |
|---|---|
| FDA Alert | Warning issued for counterfeit Ozempic found in the U.S. drug supply. |
| Counterfeit Identification | Affected drugs have lot number PAR0362 and serial numbers starting with 51746517. |
| Distribution Issues | Counterfeits were distributed outside Novo Nordisk’s authorized supply chain. |
| FDA Actions | FDA seized counterfeit products for testing on April 9, 2025. |
| Risks of Counterfeits | Counterfeit medications can be synthesized in non-medical environments, posing serious health risks. |
| Expert Opinion | Patients are advised to obtain medications only from licensed pharmacies and healthcare providers. |
Summary
Counterfeit Ozempic poses serious health risks as fake drugs circulate in the U.S. market, prompting urgent warnings from the FDA. The counterfeit medications lack proper quality assurance and can lead to harmful outcomes when used. Patients are strongly advised to verify the authenticity of their medications, ensuring they are sourced from licensed professionals to guarantee safety. The ongoing investigation by the FDA aims to eliminate these dangerous counterfeits and protect patient health.