CDC COVID Vaccine Recommendations Under Trump Administration
The CDC COVID vaccine recommendations have been a cornerstone of public health guidance throughout the pandemic, but recent developments suggest a significant policy shift. Under the Trump administration’s increased scrutiny of vaccination protocols, the Department of Health and Human Services (HHS), led by Secretary Robert F. Kennedy Jr., aims to reconsider the push for COVID-19 vaccinations among children and pregnant women. This potential withdrawal from CDC guidance—the recommendations that advocate for vaccinations starting from six months of age—marks a turning point in federal health strategies. Experts warn that this pivot could erode confidence in vaccination efforts, especially among high-risk populations, as contrasting opinions surface regarding mass vaccine mandates. With only a fraction of eligible children and pregnant women having received the latest COVID vaccine, the emerging discourse around vaccination policy will undoubtedly shape future public health efforts.
In light of shifting public health strategies, discussions surrounding CDC COVID vaccine recommendations have garnered renewed attention. The Centers for Disease Control have consistently advised vaccinations as essential for protecting against severe illness, particularly in vulnerable groups such as children and expectant mothers. With the Trump administration’s recent focus on reevaluating its vaccination protocol, the implications of this potential policy alteration raise important questions about the future of immunization practices. This new direction hints at a broader vaccination policy reformation that resonates with ongoing debates about scientific evidence and health recommendations. As society navigates these crucial decisions, it becomes imperative to consider how they may impact both individual health choices and public safety measures.
Current CDC Recommendations for COVID-19 Vaccination
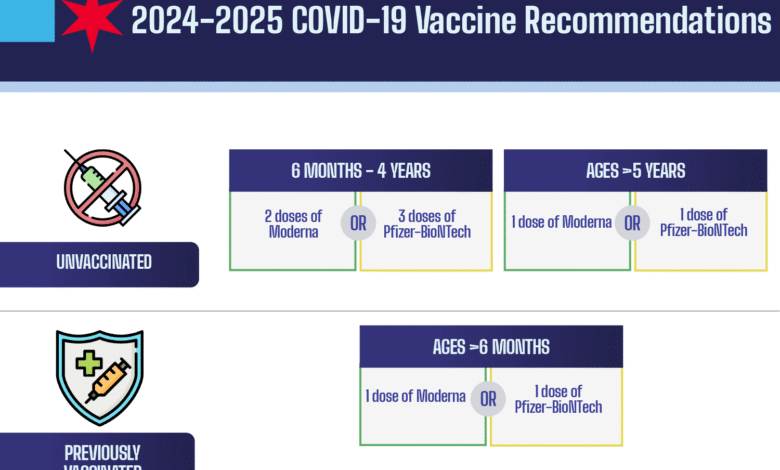
The Centers for Disease Control and Prevention (CDC) currently advocates for COVID-19 vaccination for individuals aged 6 months and older, emphasizing the importance of vaccination to curb the spread of the virus. This guidance has been a cornerstone of public health policy since the onset of the pandemic, aiming to protect vulnerable populations and achieve herd immunity. As of now, despite the CDC’s ongoing recommendations, there are reports indicating that the Trump administration, under the leadership of HHS Secretary Robert F. Kennedy Jr., is reconsidering these directives, particularly for children and pregnant women.
Vaccination rates among these groups have been notably low, with only about 13% of children and 14% of pregnant women receiving the latest COVID booster as of April. This hesitance could be further compounded by a potential shift in federal policy. If the Trump administration aligns itself more with skeptical views on vaccination, it could lead to confusion among parents and guardians about the importance of vaccinating their children, ultimately jeopardizing public health efforts.
Shift in Vaccination Policy Under Trump Administration
The Trump administration’s distancing from CDC guidance signifies a potential policy shift that could have long-lasting impacts on vaccination efforts across the United States. This policy deviation is particularly notable considering that the vaccination strategy from the earlier stages of the pandemic was driven by aggressive promotion of vaccines through initiatives like Operation Warp Speed. The anticipated changes by HHS could represent a significant pivot from aggressively promoting vaccinations to a more cautious approach, potentially affecting national immunization rates.
If Secretary Robert F. Kennedy Jr. is successful in withdrawing federal recommendations for certain groups, the message sent may further propagate vaccine hesitancy and skepticism. Such a stance aligns with Kennedy’s history of criticism regarding mRNA vaccines, which poses a risk of undermining confidence in public health directives. As critics worry about this move exacerbating already-low vaccination rates among children and pregnant individuals, supporters argue it could better align vaccination policies with scientific assessment, prioritizing informed decision-making.
Implications of the Proposed Vaccine Policy Changes
The changes in policy surrounding COVID-19 vaccinations, particularly regarding the CDC’s stance on immunizing children and pregnant women, could have far-reaching consequences for public health. One major concern raised by health experts is how this shift could dissuade individuals, especially those at high risk or with immunocompromised conditions, from getting vaccinated. Confidence in the vaccine would likely plummet if federal recommendations become inconsistent, creating an environment of uncertainty that could lead to increased transmission rates and healthcare complications.
Moreover, if insurers start to alter their coverage policies based on these recommendations, access to COVID-19 vaccines might decline, posing further threats to public health. As the FDA, led by Commissioner Dr. Marty Makary, moves towards stringent vaccine approval processes, the narrative around vaccine efficacy and the need for robust clinical data may become paramount. This friction between evolving federal policy and public health imperatives underscores the precarious nature of vaccination efforts amid ongoing pandemic challenges.
The Role of Robert F. Kennedy Jr. in Vaccine Policy Decisions
Robert F. Kennedy Jr., as the current Secretary of Health and Human Services (HHS), plays a critical role in shaping the future of vaccination policy in the United States. His well-documented skepticism towards vaccines, particularly mRNA vaccines, places him in a unique position to influence CDC guidelines significantly. Reports suggest that under his leadership, there might be a substantial shift away from the previous administration’s emphasis on mass vaccination, leading to uncertain outcomes for future public health strategies.
Kennedy’s views resonate with a significant faction of the population that questions the efficacy and safety of vaccines. As he considers amending CDC guidelines, the implications of his decisions could dramatically alter the landscape of childhood vaccinations and maternal health policies. Critics of this approach warn against potential ramifications that could arise from undermining established vaccine recommendations, highlighting the importance of relying on scientific evidence during such crucial health discussions.
Potential Impact on Immunocompromised Individuals
The proposed changes by the Trump administration regarding COVID-19 vaccination recommendations could pose significant risks, particularly for immunocompromised individuals. Should federal guidance shift to discourage vaccinations in children and pregnant women, the repercussions could extend well beyond these demographic groups. Immunocompromised patients rely heavily on community immunity to protect them from infectious diseases, and reduced vaccination rates could increase their vulnerability to COVID-19 and other illnesses.
Healthcare professionals emphasize that maintaining high vaccination rates is critical for protecting those who cannot be vaccinated for health reasons. If the administration’s policy changes discourage vaccinations, this would lead to larger outbreaks, putting lives at risk and straining the healthcare system. Thus, stakeholders from various sectors must participate in discussions that prioritize the health needs of the most vulnerable populations as the landscape of vaccination shifts under potential new guidance.
Public Responses to Vaccine Policy Changes
Public response to the Trump administration’s anticipated shift in vaccination policy has been mixed, reflecting a broader divide regarding the COVID-19 pandemic. Supporters of the administration argue that aligning vaccination policies more closely with evolving scientific understanding is essential, potentially increasing trust in health directives. However, many public health experts and proponents of vaccination view this move as a dangerous retreat from established practices that prioritize community health and safety.
As news spreads about the potential revocation of federal vaccination recommendations, parents and healthcare providers are left grappling with uncertainty regarding the best course of action. The implications of such policy changes have ignited debates in communities far and wide, with many advocating for continued commitment to vaccination as a means of ensuring public wellbeing, while others voice concerns over the transparency and integrity of the vaccination process led by the current administration.
The CDC’s Role in Informed Public Health Decisions
The CDC has historically played a crucial role in shaping public health policies, especially during health crises such as the COVID-19 pandemic. With its objective guidance grounded in scientific research, the CDC has strategically promoted vaccination as a core element of preventing widespread disease transmission. Thus, any potential changes in CDC recommendations stemming from the Trump administration’s new direction could represent a significant departure from evidence-based recommendations that the public health community has relied upon.
If the CDC’s guidance is altered or diminished under the influence of the administration, it will be essential for public health advocates to step in and emphasize the importance of following COVID-19 vaccination protocols. Maintaining communication and education about the vaccine’s efficacy and safety is critical to counteract any misinformation driven by policy changes. Trust in public health guidance hinges on transparency and scientific integrity, making it paramount for health organizations to uphold these principles amidst shifting government policy.
Healthcare Access and Vaccine Coverage
Access to healthcare and vaccination coverage is another critical dimension affected by the Trump administration’s proposed shift in COVID-19 vaccine recommendations. The possibility of insurers altering their coverage policies poses a significant threat to the availability of vaccines, particularly for marginalized and vulnerable populations. Should the administration move away from advocating for vaccinations, it could inadvertently lead to gaps in coverage that disproportionately affect those most in need of preventive care.
As experts voice concerns about the implications of these policy changes on access to vaccines, the collective effort to ensure equitable healthcare becomes even more urgent. There is a need for sustained dialogue advocating for the provision of vaccines to everyone, especially for high-risk individuals who rely on robust public health initiatives. Without adequate attention to healthcare access and insurance coverage, the intended benefits of vaccination efforts could be lost, resulting in detrimental health outcomes across communities.
Navigating the Future of COVID-19 Vaccination
As the landscape of COVID-19 vaccination faces potential upheaval with policy changes on the horizon, the future remains uncertain for public health. The Trump administration’s response to CDC recommendations could redefine how families and individuals approach vaccination. The discourse surrounding the efficacy of vaccines, particularly in children and pregnant women, may shift as the administration grapples with mixing scientific data under the scrutiny of public opinion.
To navigate this evolving landscape, it becomes increasingly vital for public health entities, medical professionals, and society as a whole to remain informed and engaged in advocacy for vaccination. Emphasizing the lasting benefits of COVID-19 vaccines, including their role in preventing severe illness and protecting public health, will be fundamental in countering any negative impacts arising from policy changes and ensuring a healthier future for all.
Frequently Asked Questions
What are the current CDC COVID vaccine recommendations for children and pregnant women?
The CDC currently recommends that everyone aged 6 months and older should receive the COVID-19 vaccine. However, there are reports suggesting a potential shift in CDC guidance influenced by the Trump administration, particularly regarding the vaccination of children and pregnant women.
How is the Trump administration influencing CDC COVID vaccine recommendations?
The Trump administration is reportedly distancing itself from CDC COVID vaccine recommendations, specifically concerning children and pregnant women. A significant policy shift may occur under HHS Secretary Robert F. Kennedy Jr., who is contemplating withdrawing federal recommendations for these groups to receive the COVID vaccine routinely.
What is Robert F. Kennedy Jr.’s role in changing CDC COVID vaccine guidelines?
As the Secretary of Health and Human Services, Robert F. Kennedy Jr. has the authority to amend CDC COVID vaccine recommendations. His known criticism of mRNA vaccines suggests that he may influence a shift in vaccination policies, potentially impacting the CDC’s guidance for vulnerable populations.
How might changes in CDC COVID vaccine recommendations affect vaccination rates?
Changes in CDC COVID vaccine recommendations, particularly if influenced by the Trump administration and Robert F. Kennedy Jr., could lead to decreased vaccination rates among children and pregnant women. Criticism arises from concerns that such a shift may discourage vaccination and increase risks for immunocompromised individuals.
What are the implications of CDC COVID vaccine recommendation changes for insurers?
If the CDC modifies its COVID vaccine recommendations, it could raise questions about whether insurers will continue to cover the vaccines. This potential shift in federal health policy may undermine prior initiatives from the Trump administration, such as Operation Warp Speed, designed to promote widespread vaccination efforts.
Why are some parents hesitant to vaccinate their children against COVID-19?
Hesitancy among parents to vaccinate their children against COVID-19 may stem from recent CDC vaccine recommendations that are being reconsidered, a lack of robust uptake (only 13% of children vaccinated as of April), and concerns regarding the implications of the Trump administration’s policy shift on vaccine safety and effectiveness.
What is the significance of the FDA’s new vaccine approval process for COVID-19 vaccines?
The FDA, under Commissioner Dr. Marty Makary, is preparing to implement a stricter approval process for COVID-19 vaccines. This aligns with emerging CDC guidance adjustments and reflects an emphasis on ensuring that vaccines are based on sound scientific evidence, which could impact public perception and uptake.
How could the changing CDC COVID vaccine recommendations affect public health policy?
A shift in CDC COVID vaccine recommendations, especially under the influence of the Trump administration and Robert F. Kennedy Jr., could lead to a major alteration in public health policy, potentially reducing the push for widespread vaccination and raising public health concerns, particularly among vulnerable groups.
What evidence supports the current CDC COVID vaccine recommendations?
The CDC’s recommendations advocate for COVID vaccination based on evidence suggesting it helps prevent severe illness and hospitalization, particularly in the context of emerging variants. However, the shifting landscape with input from the Trump administration and Robert F. Kennedy Jr. may affect how this evidence is interpreted and communicated.
What should I consider when evaluating CDC COVID vaccine recommendations amid policy changes?
When evaluating CDC COVID vaccine recommendations during this period of potential policy shifts, consider the emerging scientific evidence, the implications of statements from health officials like Robert F. Kennedy Jr., and the possible impacts on public health and personal health decisions.
| Key Points | Details |
|---|---|
| Trump Administration Distance from CDC | Plans to withdraw vaccination recommendations for children and pregnant women. |
| Current CDC Guidelines | Recommends vaccination for everyone aged 6 months and older; this may change soon. |
| Decision Impact | Potential shift in federal health policy; could affect vaccination strategy and insurance coverage. |
| Low Vaccination Rates | As of April, only 13% of children and 14% of pregnant women have received the latest vaccine. |
| HHS Secretary’s Stance | Robert F. Kennedy, Jr. has criticized mRNA vaccines and mass vaccination efforts. |
| FDA Stance | Stricter approval process for vaccines to ensure high-quality data; focus on high-risk individuals. |
Summary
The CDC COVID vaccine recommendations are currently under scrutiny as the Trump administration, via the Department of Health and Human Services, seeks to withdraw federal guidelines for vaccinating children and pregnant women against COVID-19. This potential shift could significantly alter public health policy and impact vaccination rates among vulnerable populations, raising concerns about the health implications for those at greater risk.