Alzheimer’s Treatment: Cancer Drugs May Reverse Disease Effects
Alzheimer’s treatment has taken a promising turn with recent research highlighting the potential of repurposed cancer drugs. Researchers at the University of California San Francisco (UCSF) have identified that letrozole and irinotecan – both FDA-approved drugs typically used in cancer therapy – might effectively slow or even reverse cognitive decline associated with Alzheimer’s disease. By analyzing extensive electronic medical records, the study found a significant correlation between the use of these drugs and a reduced likelihood of developing Alzheimer’s among older adults. Laboratory tests conducted on mice showed that these medications can reverse genetic changes linked to the disease and decrease the aggregation of tau proteins, factors that contribute to its progression. This groundbreaking study illuminates the potential of using existing medications, like letrozole for Alzheimer’s, to develop new strategies for effective cognitive decline treatment, potentially opening new avenues for patient care and management.
Exploring alternatives for Alzheimer’s care, researchers have turned to established cancer therapies as a potential solution for this devastating condition. The investigation into these repurposed medications focuses on how they can impact the degenerative processes of Alzheimer’s, revealing new opportunities for cognitive health management. Current knowledge about letrozole and irinotecan positions them as frontrunners in the endeavor to combat Alzheimer’s, particularly as they have already received FDA approval for other medical uses. With the increasing prevalence of Alzheimer’s disease, the need for effective treatments becomes critical, prompting scientists to seek innovative approaches in cognitive decline treatment. The findings not only raise optimism about these drugs but also encourage further exploration into the relationship between oncology and neurology.
The Discovery of Repurposed Cancer Drugs for Alzheimer’s Treatment
Recent research has unearthed the potential of repurposed cancer drugs, specifically letrozole and irinotecan, in the fight against Alzheimer’s disease. Conducted by researchers at the University of California San Francisco (UCSF), this groundbreaking study sheds light on how these FDA-approved cancer medications could not only slow cognitive decline but also reverse some of the detrimental effects of Alzheimer’s. The investigation delved into extensive electronic medical records, revealing that seniors who were treated with these drugs exhibited a significantly reduced risk of developing the disease.
In the laboratory, these cancer agents demonstrated remarkable effects on mice, reversing genetic alterations tied to Alzheimer’s and reducing tau protein clumping—one of the primary indicators of the disease. The implications of this study are profound, suggesting that existing cancer therapies may hold the key to innovative Alzheimer’s treatments. As Alzheimer’s disease is notoriously complex, the utilization of familiar, FDA-approved drugs could expedite the development of effective therapies, benefiting countless patients and their families.
Letrozole: A Promising Candidate in Alzheimer’s Treatment
Letrozole, predominantly recognized as a hormone therapy for breast cancer, has emerged as a significant player in the potential treatment of Alzheimer’s disease. Its capability to influence brain chemistry suggests that it may help mitigate cognitive decline. The UCSF study illustrates that letrozole can reverse certain genetic factors that lead to neurodegeneration, fostering a renewed understanding of how existing cancer therapies can be mobilized against Alzheimer’s. This innovative approach stands to open new avenues for Alzheimer’s treatment that are not solely reliant on traditional neurological drugs but instead capitalize on existing cancer medications.
Moreover, clinical insights revealed from the study indicated that letrozole may help to reduce inflammation in the brain, a known contributor to Alzheimer’s disease progression. Patients suffering from Alzheimer’s often experience a cascade of neuroinflammatory responses that contribute to cognitive decline. By repurposing letrozole, researchers believe they can harness its therapeutic potential to tackle these inflammatory processes, making it a promising candidate for future clinical applications in Alzheimer’s treatment.
Irinotecan: A Key Player in Alzheimer’s Research
Irinotecan, traditionally employed in cancer treatments particularly for colon and lung cancers, has surfaced as another intriguing candidate in Alzheimer’s disease strategy per recent findings from the UCSF study. Its ability to penetrate the blood-brain barrier positions irinotecan advantageously in addressing the transformative effects of the disease. As scientists continue to explore its mechanisms, early results suggest that irinotecan may help in reducing the formation of tau tangles, a hallmark of Alzheimer’s pathology.
The findings surrounding irinotecan in relation to cognitive decline treatment provide a paradigm shift in how Alzheimer’s may be addressed. With a need for more effective therapies, the potential for this FDA-approved drug to aid in alleviating symptoms presents a unique opportunity in clinical research. By integrating irinotecan in future trials, researchers hope to formally validate these promising outcomes and translate them into tangible treatment pathways for individuals battling Alzheimer’s.
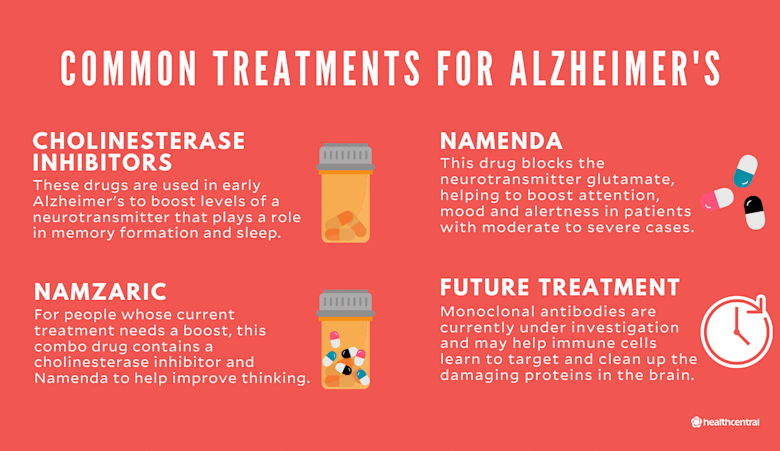
FDA-Approved Drugs: A New Hope for Alzheimer’s Patients
The evolving landscape of Alzheimer’s treatment is underscored by the recent exploration of FDA-approved drugs like letrozole and irinotecan. Currently, only two disease-modifying medications, lecanemab and donanemab, have received FDA approval, both designated for early-staged Alzheimer’s patients. This limitation has generated a pressing need for alternative therapeutic options, thereby intensifying interest in repurposing established medications. The UCSF findings contribute to a burgeoning optimism that other approved drugs could potentially alleviate the disease, addressing both cognitive and behavioral symptoms.
Beyond just offering a supplement to existing treatments, these repurposed cancer drugs may ultimately change the standard of care for Alzheimer’s patients. As the research community prioritizes rigorous clinical trials, the ability to leverage already approved substances for new indications represents a significant strategic pivot in the pursuit of ameliorating the cognitive decline associated with Alzheimer’s, reaching individuals who have previously had limited options.
The Role of Clinical Trials in Alzheimer’s Drug Repurposing
The pathway to redefining Alzheimer’s treatment through repurposed cancer drugs hinges significantly on the successful execution of clinical trials. The UCSF researchers emphasize the importance of validating their findings in human subjects to ascertain the safety and efficacy of combining letrozole and irinotecan for Alzheimer’s patients. This step is crucial not only in establishing the drugs’ therapeutic potential but also in addressing the noted variability in responses between sexes observed during preclinical studies.
Implementing rigorous clinical trials allows for a comprehensive assessment of the benefits and risks associated with these repurposed drugs. Researchers aim to evaluate their effectiveness in real-world scenarios, which is indispensable for formulating evidence-based treatment guidelines for Alzheimer’s. Furthermore, well-designed studies will incorporate demographic factors, ensuring that the treatment methods developed are inclusive and beneficial for a diverse patient population.
Cognitive Decline Treatment: Expanding the Therapeutic Arsenal
As the search for effective cognitive decline treatment options intensifies, the recent UCSF findings spotlight the urgent need to expand the therapeutic arsenal available for Alzheimer’s. Current options remain limited, with few FDA-approved drugs capable of altering the disease’s trajectory. The innovative strategy of utilizing existing medications like letrozole and irinotecan emphasizes a proactive approach to combating cognitive decline, moving away from the traditional singular focus on new drug development.
Enhancing the treatment landscape through drug repurposing not only fosters rapid innovation but also capitalizes on the existing knowledge surrounding drug interactions, side effects, and patient management. By integrating cancer medications into Alzheimer’s treatment protocols, researchers can offer hope for slowing or reversing cognitive decline while paving the way for further advancements in neurology and geriatric medicine.
Gender Differences in Drug Response: Implications for Alzheimer’s Treatment
An intriguing aspect of the UCSF study highlights the gender differences observed in the response to letrozole and irinotecan, particularly noting that male mice exhibited more favorable outcomes than their female counterparts. These findings shed light on a critical consideration in Alzheimer’s research, emphasizing that treatments may not affect all patients equally. Such disparities in treatment response underscore the necessity for tailored approaches to Alzheimer’s therapy, which recognizes the importance of gender and biological diversity in drug efficacy.
Understanding these differences is pivotal for developing comprehensive treatment strategies for Alzheimer’s patients. As research progresses, it is essential to consider how varying biological responses to medications can influence clinical outcomes. Addressing gender disparities is fundamental not only in the design of future trials but also in ensuring equitable access to effective Alzheimer’s treatments for all patients.
Future Directions in Alzheimer’s Research: Challenges and Opportunities
The landscape of Alzheimer’s research is evolving swiftly, with new opportunities emerging from innovative studies like the one undertaken at UCSF. The potential for repurposing FDA-approved cancer drugs opens up new directions in the quest for effective Alzheimer’s therapies. However, challenges remain in conducting human trials, as the complexities and variations in disease presentation make the transition from animal models to human subjects fraught with difficulties.
Researchers are now tasked with translating their findings into actionable treatments while facing regulatory hurdles and the need for comprehensive evaluation regarding safety and efficacy. Nevertheless, the momentum generated by these discoveries could lead to accelerated pathways for drug approval, providing Alzheimer’s patients with novel therapeutic options that may improve their quality of life and survival outcomes.
Innovative Approaches to Alzheimer’s Treatment Simplified
In summary, the exploration of repurposed cancer drugs like letrozole and irinotecan provides a simplified yet innovative approach to Alzheimer’s treatment. The preliminary findings offer a glimpse of hope, indicating potential pathways to developing effective therapies that slow or even reverse cognitive decline. The shift towards utilizing well-established drugs may expedite the process of finding accessible treatments for a disease that has long resisted effective intervention.
This burgeoning area of research is inviting a collaborative effort from the medical community, regulatory bodies, and patients alike, reinforcing the idea that innovative therapies can emerge from seemingly unrelated fields. As these studies progress, they hold promises not just for patients currently battling Alzheimer’s, but also for future generations who may face this daunting challenge.
Frequently Asked Questions
What are the potential effects of repurposed cancer drugs like letrozole for Alzheimer’s treatment?
Recent research indicates that repurposed cancer drugs such as letrozole may have the potential to slow down or reverse the effects of Alzheimer’s disease. Studies show that letrozole, commonly used for breast cancer, can help in reducing tau protein clumping, which is linked to cognitive decline.
How does irinotecan relate to Alzheimer’s treatment studies?
Irinotecan, a drug used for colon and lung cancers, has been studied for its potential in Alzheimer’s treatment. The findings suggest that this drug, when used in combination with letrozole, can reverse genetic changes associated with Alzheimer’s, thus promising for cognitive decline treatment.
What FDA-approved drugs for Alzheimer’s disease are currently available?
As of now, there are two FDA-approved drugs for Alzheimer’s treatment: lecanemab and donanemab. These medications are disease-modifying therapies but are specifically approved for use in early-stage Alzheimer’s patients.
What are the implications of combining letrozole and irinotecan for Alzheimer’s treatment?
The combination of letrozole and irinotecan presents a novel approach to Alzheimer’s treatment, as initial studies have shown that this pairing can reverse some of the harmful brain changes associated with the disease. Clinical trials are needed to assess this further.
What limitations exist in the studies on Alzheimer’s treatment with letrozole and irinotecan?
The current studies on the potential of letrozole and irinotecan in treating Alzheimer’s highlight a need for further validation through human trials. Additionally, there are noted gender differences in response to these drugs, which could affect treatment effectiveness.
Why is there hope for Alzheimer’s treatment through cancer drug repurposing?
Repurposing FDA-approved cancer drugs like letrozole and irinotecan for Alzheimer’s treatment offers hope due to their established safety profiles and the potential for rapid clinical application. The study from UCSF suggests that these medications could effectively address complex brain changes associated with Alzheimer’s.
What role do FDA-approved drugs play in Alzheimer’s cognitive decline treatment?
FDA-approved drugs serve a critical role in Alzheimer’s cognitive decline treatment by providing options that have undergone rigorous testing for safety and efficacy. However, ongoing research into repurposed medications like letrozole and irinotecan can expand these treatment options.
What are the next steps in Alzheimer’s research concerning cancer drugs?
The next steps involve conducting clinical trials to explore the combined use of letrozole and irinotecan in patients with Alzheimer’s disease. This research aims to validate findings from animal studies and assess the efficacy of these repurposed drugs in human patients.
| Key Point | Details |
|---|---|
| Researchers’ Discovery | Two cancer drugs, letrozole and irinotecan, may slow down or reverse Alzheimer’s disease. |
| Study Methodology | Analysis of medical records from individuals over 65 revealed lower Alzheimer’s risk in those using these drugs. |
| Drug Applications | Letrozole is used for breast cancer, and irinotecan for colon and lung cancers. |
| Laboratory Results | In mice, the drug combination reversed genetic changes associated with Alzheimer’s and reduced tau protein clumping. |
| Significance of the Study | Utilizing existing FDA-approved medications offers an innovative approach to Alzheimer’s treatment. |
| Expert Opinion | Dr. Marina Sirota mentions that the complexity of Alzheimer’s has made treatment challenging, but new tools offer hope. |
| Limitations | Further validation through human trials is needed; gender differences in drug response were also noted. |
| Current Treatments | Only two FDA-approved medications for Alzheimer’s exist for early-stage patients: lecanemab and donanemab. |
| Future Directions | Plans to initiate clinical trials for letrozole and irinotecan combined effects in humans with Alzheimer’s. |
Summary
Alzheimer’s treatment has taken a promising turn with the discovery that two existing cancer medications, letrozole and irinotecan, could potentially slow down or reverse the effects of this debilitating disease. Research from UCSF highlights the innovative repurposing of these FDA-approved drugs, showing their ability to reverse genetic changes and reduce tau protein clumping in laboratory settings. While further trials are necessary to confirm these findings in humans, this approach could offer new hope in the fight against Alzheimer’s, especially considering the limited options currently available for patients.