Alzheimer’s Blood Test: FDA Approves New Diagnostic Tool
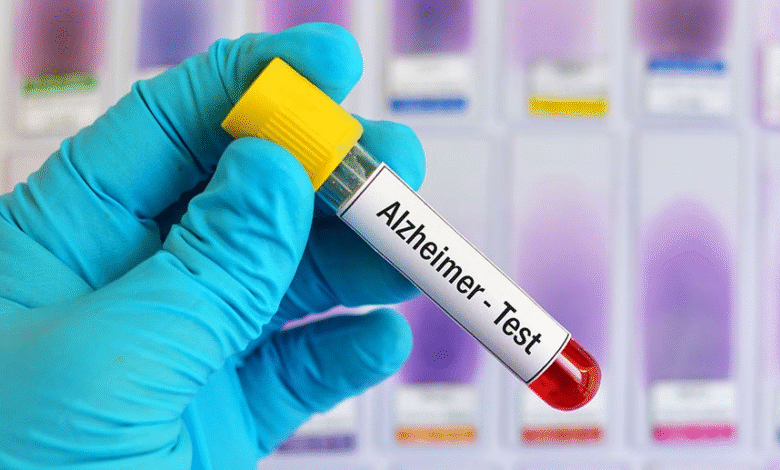
The recently introduced Alzheimer’s blood test has made headlines with its groundbreaking FDA approval, offering a new avenue for early detection of Alzheimer’s disease. This innovative Lumipulse Alzheimer test is the first in-vitro diagnostic device specifically designed to detect amyloid plaques—key indicators of Alzheimer’s—within a simple blood sample. With the increasing prevalence of Alzheimer’s, affecting more than 6 million Americans, this early detection Alzheimer’s test can significantly enhance diagnostic accuracy and patient management. Unlike traditional methods like PET scans, which are costly and invasive, the Lumipulse test provides a quick and non-invasive alternative for patients over the age of 55. As Alzheimer’s diagnosis innovation advances, this blood test could change the landscape of how we approach this complex disease, providing hope for millions and potentially reducing the burden on healthcare systems.
The recent FDA approval of a blood-based diagnostic for Alzheimer’s marks a pivotal moment in the fight against this life-altering condition. With terms like Alzheimer’s screening, cognitive impairment assessment, and amyloid detection gaining traction, the Lumipulse test represents a significant innovation in medical diagnostics. Instead of relying on more invasive procedures, the ability to quickly test for amyloid plaques through a simple blood draw offers a less daunting alternative for patients. As awareness grows about Alzheimer’s and its implications, early detection methods like this blood test will likely play a crucial role in patient care. Moreover, such advancements not only foster hope among patients but also facilitate a proactive approach in managing this progressive disorder.
FDA Approval Marks a New Era in Alzheimer’s Testing
The recent approval of the Lumipulse Alzheimer’s blood test by the FDA signifies a groundbreaking moment in the fight against Alzheimer’s disease. This innovative diagnostic tool allows for the early detection of the disease in adults over 55, who present cognitive symptoms. Before its approval, patients often had to rely on more invasive methods such as PET scans to identify amyloid plaques, which are indicative of Alzheimer’s pathology. The Lumipulse test provides a less invasive, cost-effective alternative while streamlining the diagnosis process.
The FDA has recognized the importance of this advancement, especially in light of the rising number of Alzheimer’s cases projected to reach nearly 13 million by 2050. Early detection is crucial as it enables timely intervention, which can potentially alter the course of the disease. With the Lumipulse device, healthcare providers can more effectively manage treatment options, making it a vital tool in Alzheimer diagnosis innovation.
Understanding the Lumipulse Alzheimer Test
The Lumipulse Alzheimer test leverages cutting-edge technology to detect amyloid plaques in the bloodstream, which are essential biomarkers for diagnosing Alzheimer’s disease. This approach enhances the ability to discern Alzheimer’s pathology from standard cognitive assessments that may overlook subtle changes. The clinical study backing the test demonstrated a remarkable 91.7% accuracy in detecting amyloid plaques in cognitively impaired individuals, showcasing its potential as a robust screening tool.
Unlike traditional diagnostic methods, the Lumipulse test offers a quicker and less expensive alternative, ultimately reducing the burden on patients and healthcare systems. Although the FDA has warned about the risk of false positives, the breakthrough provides significant hope for early detection Alzheimer’s initiatives, ensuring that patients can receive timely care without the complications associated with invasive procedures.
The Importance of Early Detection in Alzheimer’s Care
Early detection of Alzheimer’s disease is crucial for effective management and treatment. With the introduction of the Lumipulse blood test, individuals who exhibit cognitive symptoms can be diagnosed more efficiently, allowing for prompt intervention. This proactive approach can lead to better outcomes, as treatments can be administered when the disease is at an earlier stage. Furthermore, early detection not only aids in patient care but also empowers families to prepare and make informed decisions about future healthcare needs.
The significance of early detection is underscored by alarming statistics: approximately 10% of individuals aged 65 and older have Alzheimer’s, a figure expected to double within the coming decades. By utilizing tools like the Lumipulse test, healthcare professionals can revolutionize Alzheimer’s care, ensuring that those at risk receive the support and treatment necessary to manage their condition effectively.
How Lumipulse Changes Alzheimer’s Diagnosis
The Lumipulse device is set to change the landscape of Alzheimer’s diagnosis significantly. This non-invasive blood test not only provides a reliable means of detecting amyloid plaques but also reduces the financial and emotional strain on patients who would otherwise undergo complicated imaging tests. The FDA’s approval of this technology marks a pivotal shift in how Alzheimer’s disease is diagnosed, focusing on accessibility and efficiency.
The test works by analyzing blood samples for specific proteins that indicate the presence of neurological disease. Enhanced accessibility and affordability mean that more people can undergo testing, leading to earlier interventions and better management plans. As more healthcare providers adopt the Lumipulse Alzheimer test, the vision of improving early detection Alzheimer’s strategies will become a reality, ultimately benefiting millions.
Potential Risks Associated with the Lumipulse Alzheimer Test
While the Lumipulse Alzheimer test presents cutting-edge technology for early dementia detection, potential risks remain. The FDA has issued warnings regarding possible false positives, which may lead to misdiagnosis and inappropriate treatment plans. It is essential for both patients and healthcare professionals to understand these risks fully, as inaccurate results can create unnecessary anxiety and possibly result in harmful treatment decisions.
Thus, while the Lumipulse test signifies a remarkable advancement in Alzheimer’s diagnosis innovation, it must be utilized responsibly. Follow-up consultations and thorough clinical evaluations are vital to ensuring that diagnostic accuracy is maintained. Educating healthcare providers about the implications of false results and implementing additional confirmatory tests when necessary will help mitigate these risks.
Impact of FDA Approval on Alzheimer’s Research
The FDA’s approval of the Lumipulse blood test not only affects clinical practice but also revitalizes research opportunities in Alzheimer’s disease. Researchers can now explore further avenues for understanding amyloid pathology with accessible blood-based biomarkers, leading to a potentially deeper understanding of Alzheimer’s progression and treatment responses. This advancement helps to lay the groundwork for future innovations in Alzheimer’s diagnostics, emphasizing the importance of a multidisciplinary approach to tackling the disease.
Moreover, this approval could pave the way for further financial investment into Alzheimer’s research as companies and institutions recognize the potential for therapeutic interventions based on early detection. By enhancing our understanding of the disease’s nuances through blood tests, researchers and healthcare professionals can devise targeted therapies, improving outcomes for millions of Alzheimer’s patients and their families.
The Future of Alzheimer’s Testing
With the introduction of the Lumipulse Alzheimer test, the future of Alzheimer’s testing appears much brighter. This breakthrough signals the need for continuous innovation in diagnostics that can lead to more accurate assessments of cognitive decline. As technology continues to progress, researchers are optimistic about developing even more sophisticated testing protocols that marry convenience with scientific rigor. The quest for improved Alzheimer diagnosis innovation is set to shape the landscape of patient care for years to come.
As awareness increases surrounding Alzheimer’s risks and available testing options, it is likely that more patients will seek early testing. Recent statistics show that almost seven million Americans currently live with Alzheimer’s, marking a critical time to adopt new detection methods like Lumipulse that promote early intervention. With ongoing support from the different sectors of healthcare, it is plausible that advances in Alzheimer’s diagnostics will play a central role in changing how we address this pervasive issue.
Challenges Facing the Adoption of New Alzheimer Tests
Despite the promising nature of the Lumipulse Alzheimer test, challenges remain in its widespread adoption in clinical settings. Healthcare systems must adapt to incorporate new technologies, which often requires additional training for medical staff and potentially lengthy changes in administrative protocols. Moreover, variability in insurance coverage for this test could limit access for many patients, ultimately impacting the overall effectiveness of early detection protocols that benefit from such innovations.
Furthermore, there is the challenge of public perception. Many patients may have apprehensions regarding new diagnostic methods, particularly related to accuracy and implications of false positives. Educating both healthcare providers and patients about Lumipulse’s functionality, its role in early detection, and its limitations will be essential for facilitating acceptance and integration into regular practice. Overcoming these barriers is key to ensuring that the advancements in Alzheimer diagnosis become available to those who need them the most.
Supporting Patients Through the Alzheimer’s Diagnosis Process
Navigating an Alzheimer’s diagnosis can be an overwhelming experience for patients and their families. With the newfound capability to utilize the Lumipulse blood test, healthcare providers must prioritize transparent communication throughout the testing and diagnostic process. Providing clear explanations about what the test entails, its risks, and how results will be interpreted is essential for alleviating anxiety and fostering a supportive environment.
Moreover, after the results are received, it is pivotal for healthcare practitioners to provide follow-up consultations. These discussions are vital for interpreting the implications of test results, addressing any concerns regarding potential treatment pathways, and fostering a holistic understanding of the patient’s condition. By ensuring thorough support throughout this journey, healthcare providers can help empower patients and families, guiding them toward the most appropriate care plans.
Frequently Asked Questions
What is the FDA approval for the Alzheimer’s blood test?
The FDA approved Lumipulse, the first-ever blood test specifically designed for the early detection of Alzheimer’s disease in adults over 55 showing symptoms. This in-vitro diagnostic device offers a quicker and less invasive alternative to traditional PET scans for identifying amyloid plaques, which are crucial indicators of Alzheimer’s.
How does the Lumipulse Alzheimer test work?
The Lumipulse Alzheimer test works by detecting amyloid plaques in the blood, which are associated with Alzheimer’s disease. In clinical studies, it has shown a 91.7% accuracy rate in identifying these plaques among cognitively impaired adults, providing a reliable assessment of Alzheimer’s pathology.
What are the benefits of the early detection Alzheimer’s blood test?
The early detection Alzheimer’s blood test, specifically the Lumipulse test, offers several benefits. It is cost-effective and quicker compared to PET scans, reduces exposure to radiation, and can help healthcare providers make informed decisions regarding diagnosis and treatment, ultimately improving care for patients showing early signs of Alzheimer’s.
Can the Lumipulse test replace PET scans for Alzheimer’s diagnosis?
While the Lumipulse test presents a significant advancement in diagnosing Alzheimer’s, it may not completely replace PET scans. However, it can serve as a preliminary screening tool to determine the necessity of more invasive tests, potentially streamlining the diagnostic process for Alzheimer’s.
What should patients know about the accuracy of the Alzheimer’s blood test?
Patients should be aware that while the Lumipulse test has shown high accuracy in clinical studies for detecting amyloid plaques, there are risks of false positives. This means that incorrect diagnoses could occur, making it essential for patients to discuss results and any further steps with their healthcare provider.
What is the significance of the Lumipulse Alzheimer test in Alzheimer’s diagnosis innovation?
The Lumipulse Alzheimer test marks a significant breakthrough in Alzheimer’s diagnosis innovation, as it provides a non-invasive and efficient method to detect amyloid pathology. This advancement is particularly important given the increasing number of Alzheimer’s cases, as it offers hope for early intervention and management of the disease.
What are amyloid plaques and why are they important in Alzheimer’s blood tests?
Amyloid plaques are clumps of protein that accumulate in the brains of individuals with Alzheimer’s disease and are considered key pathological markers. Their detection through blood tests like Lumipulse is crucial because it can aid in confirming an Alzheimer’s diagnosis early on, allowing for timely treatment options.
Who is the target demographic for the Lumipulse Alzheimer test?
The target demographic for the Lumipulse Alzheimer test includes adults over the age of 55 who are exhibiting signs and symptoms of Alzheimer’s disease. This test aims to facilitate early detection, which is vital for effective management and intervention.
| Key Points |
|---|
| FDA approves first blood test for Alzheimer’s disease (Lumipulse). |
| Designed for early detection in adults over 55 with symptoms. |
| Detects amyloid plaques, a key indicator of Alzheimer’s disease. |
| Offers a cost-effective alternative to PET scans which involve radiation. |
| Clinical study shows 91.7% accuracy in identifying amyloid plaques. |
| Warning: Potential for false positives leading to misdiagnosis. |
| There are nearly 7 million Americans currently living with Alzheimer’s; projected to rise to 13 million by 2050. |
| Significant advancement in Alzheimer’s diagnosis accessibility for U.S. patients. |
Summary
The advancement of the Alzheimer’s blood test represents a groundbreaking achievement in the early detection of Alzheimer’s disease. This new test, Lumipulse, provides a non-invasive, cost-effective method for identifying the presence of amyloid plaques in patients over the age of 55 who exhibit symptoms of this debilitating condition. With its 91.7% accuracy rate demonstrated in clinical studies, the test is poised to revolutionize how Alzheimer’s disease is diagnosed, making it more accessible to those in need and potentially transforming the standard of care for millions affected by Alzheimer’s.