Chikungunya Vaccine: Seniors Warned About Risks Before Travel
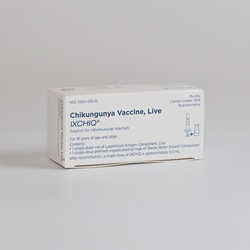
The chikungunya vaccine, known as Ixchiq, represents a pivotal development in controlling the mosquito-borne chikungunya virus, particularly among travelers. Approved by the FDA in November 2023, this vaccine marks a significant breakthrough in public health, offering hope against a virus that has plagued many regions worldwide. However, recent advisories highlight potential concerns, particularly for older adults who are warned of serious complications, including fatalities linked to the vaccine. As these individuals prepare for travel, understanding both chikungunya symptoms and vaccine safety is crucial. The CDC continues to monitor the situation, emphasizing the importance of informed health choices as senior travel health becomes more relevant in today’s global landscape.
The Ixchiq vaccination, aimed at preventing chikungunya infection, has recently garnered attention for its implications in senior health, particularly for those planning to travel. With chikungunya characterized by debilitating symptoms like fever and severe joint pain, staying informed on vaccine safety and potential risks is urgent for older travelers. The recent warnings from health authorities underscore the nuanced balance travelers must navigate between protection and risk, specifically regarding the chikungunya vaccine. As public health officials strive to ensure the FDA chikungunya approval process remains vigilant, travelers are encouraged to stay updated on any changes. Recognizing the importance of safe travel health practices will not only aid in avoiding chikungunya but also enhance overall well-being during adventures abroad.
Understanding the Ixchiq Vaccine and Its Approval
The Ixchiq vaccine, developed by Valneva, made headlines after gaining FDA approval in November 2023. This marked a significant milestone in public health, as it is the first vaccine specifically targeting the chikungunya virus, a dangerous mosquito-borne illness. The approval allows the Ixchiq vaccine to be administered to individuals aged 18 and older, particularly those who might be traveling to regions where the virus is prevalent. It is crucial for travelers to understand not only the benefits of getting vaccinated but also the associated risks, particularly in older adults.
As chikungunya cases continue to rise globally, the FDA’s endorsement of the Ixchiq vaccine underscored its importance in preventing the disease. However, the regulatory body has since issued warnings about the vaccine’s usage among older populations. This decision stems from concerns about serious adverse events, highlighting the necessity for healthcare providers to offer comprehensive counseling regarding vaccination, especially for senior travelers who may have underlying health issues.
Safety Concerns Regarding the Chikungunya Vaccine
Recent safety advisories from the FDA and CDC highlight alarming reports of severe reactions following the administration of the Ixchiq vaccine in individuals over 60. There have been instances of neurological and cardiac complications, including deadly outcomes attributed to the vaccine. As a result, the agencies recommend pausing the vaccine’s usage for this demographic until further evaluations can provide clarity on the risks. Senior travelers must be acutely aware of such warnings to make informed decisions about their health.
The CDC’s notice comes in the wake of documented adverse effects that resemble the chikungunya symptoms themselves, such as fever and severe joint pain. These reactions could mislead elderly patients into confusing vaccine side effects with the actual illness, complicating treatment and recovery efforts. The current recommendation emphasizes the necessity of thorough discussions between healthcare providers and patients about the potential dangers before considering the chikungunya vaccine.
Chikungunya Symptoms: What to Watch For
Chikungunya is characterized by a range of symptoms that often include debilitating joint pain, fever, headaches, and rashes. While many individuals recover within a week, a subset may experience prolonged and severe symptoms that can disrupt their daily lives. Given that the Ixchiq vaccine may also elicit symptoms resembling those of chikungunya, it is vital for travelers, especially seniors, to differentiate between vaccine side effects and actual chikungunya infection. Understanding these symptoms can lead to timely medical intervention and better health outcomes.
For older adults at risk, awareness of chikungunya symptoms is paramount, particularly when traveling to endemic regions. The CDC has provided guidance on how to identify these symptoms promptly, enabling individuals to seek medical attention swiftly. This heightened vigilance can be particularly beneficial for seniors, who are more susceptible to severe disease manifestations, reinforcing the importance of travel health preparations.
The Impact of Recent Adverse Events on Vaccine Perception
The recently reported adverse events related to the Ixchiq vaccine have generated a ripple effect on public perception concerning vaccine safety. As concerns grow about serious complications stemming from the vaccine, many potential recipients are left hesitant, especially among older populations who may already face significant health challenges. Vaccine hesitancy can have profound implications, potentially undermining broader public health goals aimed at controlling chikungunya outbreaks.
The response from regulatory bodies following these adverse events is crucial in rebuilding trust in the vaccination process. Ongoing safety evaluations by the FDA and CDC are a step towards ensuring that potential risks are communicated transparently, allowing individuals to make informed health decisions. Addressing vaccine safety comprehensively is essential for restoring confidence in public health initiatives targeting mosquito-borne viruses such as chikungunya.
Travel Health Guidelines for Seniors
As travel resumes post-pandemic, the emphasis on senior travel health has never been more critical. Older adults are often at a heightened risk for complications from diseases like chikungunya, making pre-travel consultations with healthcare providers crucial. Discussions should encompass not just vaccine options but also preventive measures against mosquito bites, such as using repellents and protective clothing, to mitigate the risk of contracting chikungunya virus while traveling.
In light of the recent advisory surrounding the Ixchiq vaccine, it is imperative that seniors evaluate their health status and travel itinerary closely. Tailored travel health plans, which encompass an understanding of potential exposure to chikungunya and its symptoms, can significantly reduce the risk of adverse health outcomes. Ensuring that older travelers are equipped with knowledge and resources can enhance their travel experience while safeguarding their health.
Next Steps Following FDA and CDC Warnings
In response to the recent warnings issued by the FDA and CDC regarding the Ixchiq vaccine, it is essential for senior travelers to stay informed about ongoing developments. As the safety evaluation continues, patience and vigilance are critical. Seniors should monitor updates from the CDC for useful guidance and consider alternatives to vaccination, especially if traveling to areas with a high incidence of chikungunya.
Engaging with healthcare providers will also be vital in navigating the next steps. Accurate and updated information can empower older adults to make sound decisions regarding their travel plans and vaccination status. Such proactive engagement not only aids in personal safety but contributes to a broader understanding of medical recommendations and public health safety.
Preventing Chikungunya: A Combined Approach
The prevention of chikungunya necessitates a multi-faceted approach, combining vaccination and protective measures against mosquito bites. While the Ixchiq vaccine is designed to provide immunity, it is equally important for seniors and travelers to implement additional strategies to reduce their risk of exposure. This includes using mosquito repellents, wearing long sleeves, and ensuring accommodations are equipped with screens or air conditioning to deter mosquito activity.
Education plays a vital role in preventing chikungunya, particularly for older travelers unaccustomed to such viruses. Informative sessions that cover both the chikungunya vaccine and effective mosquito prevention techniques can significantly enhance awareness and readiness among seniors. This proactive tandem strategy can help mitigate the impact of chikungunya, ensuring safer travel experiences.
Healthcare Provider Roles in Chikungunya Prevention
Healthcare providers play a pivotal role in informing the public about the chikungunya vaccine and associated health risks. With growing concerns regarding the Ixchiq vaccine, it is crucial for healthcare professionals to stay updated and communicate forthcoming developments to their patients clearly. Furthermore, they must assess and discuss each patient’s individual risks and benefits regarding vaccination, particularly for seniors who may face heightened health vulnerabilities.
In addition to vaccine discussions, healthcare providers should educate patients on travel-related health risks. By offering pre-travel consultations, practitioners can guide older adults on necessary precautions, vaccination options, and symptom recognition to prepare them better for potential health challenges while traveling. This partnership between healthcare providers and patients is essential in managing the health of senior travelers and facilitating safer travel experiences.
The Future of Chikungunya Vaccination and Public Health
As the chikungunya vaccine landscape evolves, ongoing research and monitoring remain critical in addressing vaccine-related safety issues. Continuous evaluations by the FDA and CDC will likely refine guidelines and recommendations for the Ixchiq vaccine in different age groups. Such diligence is essential to ensuring that the benefits of vaccination outweigh the risks and that public health initiatives remain effective in controlling chikungunya outbreaks.
Looking ahead, there is significant potential for advancements in the development of safer vaccines and treatments for chikungunya. With rising global awareness about mosquito-borne diseases, increased funding and research could lead to innovative solutions that mitigate the impact of viruses like chikungunya on vulnerable populations, including seniors. The integration of research, safety monitoring, and public education will be fundamental in shaping the future of chikungunya vaccination and protection.
Frequently Asked Questions
What is the chikungunya vaccine, Ixchiq, and how does it work?
The chikungunya vaccine, known as Ixchiq, is the first FDA-approved vaccine developed to prevent chikungunya virus, a mosquito-borne illness. It employs a live, weakened version of the virus to stimulate the immune system, helping individuals build resistance to potential future infections.
Why are older adults advised to pause the use of the chikungunya vaccine?
The FDA and CDC have recommended that adults over 60 pause the use of the chikungunya vaccine due to safety concerns. Reports of serious adverse events, including neurologic and cardiac complications, have raised alarms, particularly after two deaths were linked to the vaccine.
What are common symptoms of chikungunya that the Ixchiq vaccine might mimic?
Common symptoms of chikungunya include fever, severe joint pain, headache, muscle pain, and rash. These symptoms may also appear in some individuals after receiving the Ixchiq vaccine, which is derived from a weakened form of the virus.
When was the chikungunya vaccine approved by the FDA?
The chikungunya vaccine, Ixchiq, was approved by the FDA in November 2023, making it the first vaccine available to protect against the chikungunya virus, especially for individuals at risk of exposure.
What steps is the FDA taking to ensure the safety of the chikungunya vaccine for seniors?
The FDA is conducting an updated benefit-risk assessment for the chikungunya vaccine, Ixchiq, specifically for individuals over 60 years of age, following reports of serious adverse events in this demographic.
What should senior travelers know about chikungunya symptoms before traveling?
Senior travelers should be aware that chikungunya symptoms include fever and joint pain, which can lead to severe and long-lasting discomfort. It’s crucial to understand these symptoms as they can also be mimicked by the chikungunya vaccine, Ixchiq.
What has the CDC said regarding the safety of the Ixchiq vaccine for older adults?
The CDC urged older adults to pause the use of the Ixchiq vaccine due to ongoing safety evaluations, following reports of significant complications that may arise from vaccination in this age group.
How are chikungunya and similar viruses transmitted?
Chikungunya, along with dengue and Zika viruses, is transmitted through the bite of infected mosquitoes. This is important for travelers to understand when considering vaccination with Ixchiq.
Is the chikungunya vaccine necessary for everyone traveling to areas with chikungunya outbreaks?
While the chikungunya vaccine can be beneficial for those at risk of exposure, older adults should consult healthcare providers regarding safety and necessity, especially considering recent advisories concerning the Ixchiq vaccine.
What should individuals do if they experience adverse reactions after receiving the chikungunya vaccine?
Individuals who experience any adverse reactions after receiving the chikungunya vaccine, Ixchiq, should seek medical attention immediately and report their symptoms to a healthcare professional, as well as to the FDA.
| Key Points |
|---|
| The Ixchiq vaccination was approved by the FDA in November 2023 as the first chikungunya vaccine. |
| Older adults (60+) are advised to pause vaccination due to safety concerns and reports of serious adverse events. |
| Among adverse events, there were reports of neurological and cardiac issues, with two deaths linked to the vaccine. |
| Chikungunya is transmitted by infected mosquitoes, causing fever and severe joint pain among other symptoms. |
| The FDA will reassess the benefit-risk situation of the Ixchiq vaccine for older adults in light of recent findings. |
Summary
The chikungunya vaccine, Ixchiq, has garnered significant attention following advisory warnings from health authorities regarding its use in older adults. As concerns over safety and possible severe complications arise, particularly for individuals over 60, travelers are urged to reconsider getting vaccinated before their journeys. With the FDA and CDC actively monitoring reports and conducting risk assessments, it’s crucial for travelers to stay informed about the chikungunya vaccine and its implications for health security.